Summary
Rosuvastatin was found to have no significant benefit in the prevention of cardiovascular death, myocardial infarction, or stroke in symptomatic older patients with systolic heart failure of ischemic etiology in the Controlled Rosuvastatin Multinational [CORONA] trial. However, statin therapy was associated with significantly fewer hospitalizations and significantly decreased levels of low-density lipoprotein compared with placebo.
- heart failure clinical trials
Rosuvastatin was found to have no significant benefit in the prevention of cardiovascular (CV) death, myocardial infarction (MI), or stroke in symptomatic older patients with systolic heart failure (HF) of ischemic etiology in the Controlled Rosuvastatin Multinational (CORONA) trial. However, statin therapy was associated with significantly fewer hospitalizations and significantly decreased levels of low-density lipoprotein (LDL) compared with placebo.
Assuming that rosuvastatin reduced the risk of acute atherothrombotic events, our results suggest that the major etiology of CV deaths in these older patients with advanced systolic HF may be a primary electrical event related to ventricular dilatation and scarring and not to an atherothrombotic event, said Åke Hjalmarson, MD, PhD, Göteborg University, Sweden, who reported on the study.
CORONA enrolled 5,011 patients (24% women) with systolic HF of ischemic etiology. The mean age was 73 years. All patients were receiving optimal HF therapy. After a placebo run-in phase of 2–4 weeks, patients were randomly assigned to a daily dose of 10 mg of rosuvastatin (2,514 patients) or to placebo (2,497 patients). The median follow-up was 2.7 years.
Baseline mean LDL levels decreased from 137 mg/dL to 76 mg/dL after 3 months of treatment with rosuvastatin but did not change significantly in the placebo group (136 -< 138 mg/dL). Rosuvastatin also had a significant effect on the level of high-sensitivity C-reactive protein; the level decreased from 3.1 mg/L to 2.1 mg/L after 3 months of treatment; this 32% decrease compared with a 5% increase in the placebo group (from 3.0 mg/L at baseline to 3.3 mg/L at 3 months; p<0.001).
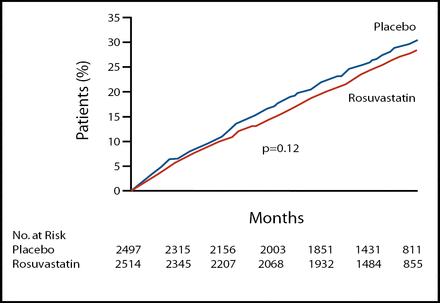
Dr. Hjalmerson reported that the incidence of the primary endpoint, a composite of CV death, nonfatal MI, or nonfatal stroke did not differ significantly between the two groups (27.5% for rosuvastatin vs 29.3% for placebo, p=0.12) (Figure 1). He noted, “The study was powered to detect a mean relative risk reduction of 16%, but the reduction associated with rosuvastatin was only 8%.”
Kaplan-Meier Estimates for the Primary Outcome, Death From Any Cause and Any Coronary Event.
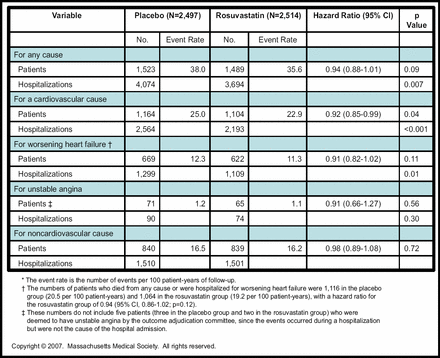
Rosuvastatin also had no significant benefit in terms of the secondary endpoints of all-cause mortality (hazard ratio (HR) 0.95; p=0.31) or any coronary event (HR 0.92; p=0.18). Cardiovascular deaths accounted for 68% of the events, and post hoc analysis indicated that rosuvastatin reduced nonfatal events (MI or stroke) by 16% (10.6% for placebo vs 9.0% for rosuvastatin; p=0.05).
Rosuvastatin was associated with significantly fewer hospitalizations for all causes, as well as for CV causes and heart failure, but not for hospitalizations related to unstable angina or non-CV causes (Figure 2).
Patients Who Had at Least One Hospitalization, and the Total Number of Hospitalizations.
Lastly, rosuvastatin did not improve patients' perception of health status, as assessed by the New York Heart Association class of heart failure or by the McMaster Overall Treatment Evaluation questionnaire, both of which were prespecified tertiary outcomes.
Previous studies have suggested that low lipid levels may be harmful in patients with heart failure, but there was no evidence of harm associated with rosuvastatin in CORONA. Rosuvastatin was well tolerated, and in fact, more patients discontinued placebo than rosuvastatin because of adverse events (302 vs 241; p=0.004). Importantly, the rates of ALT elevation and muscle-related side effects were similar between groups.
Several potential explanations have been raised for why rosuvastatin failed to meet the primary endpoint despite prior promising studies with other statins, including competing risks for events that were not modifiable by a statin; a patient population that had high comorbidity; mandated use of optimal, evidence-based treatments for heart failure; and differences in pleiotropic effects between statins (or doses). Further large, prospective studies are needed to answer the questions raised by the CORONA trial and to better delineate the role for statin therapy for patients with systolic heart failure.
The findings of this study have been published: [Kjekshus et al. NEJM 2007;357:2248–2261].
- © 2007 MD Conference Express