Summary
Intracoronary injection of skeletal muscle stem cells within days of myocardial infarction increases heart function after 1 year, according to findings of the Catheter-Based Delivery of Autologous Skeletal Myoblasts for Ischemic Cardiomyopathy: Feasibility, Safety and Improvement in Cardiac Performance [CAuSMIC] trial.
- cardiology clinical trials genomics
- myocardial infarction
Skeletal Stem Cells Improve Heart Function
Intracoronary (IC) injection of skeletal muscle stem cells within days of myocardial infarction (MI) increases heart function after 1 year, according to findings of the Catheter-Based Delivery of Autologous Skeletal Myoblasts for Ischemic Cardiomyopathy: Feasibility, Safety and Improvement in Cardiac Performance (CAuSMIC) trial.
In the open-label, phase 1 trial, 23 patients with ischemic cardiomyopathy and EF ≤40% were randomly assigned to treatment with optimal medical therapy (OMT) alone (n=11) or OMT plus autologous skeletal myoblast (ASM) transplantation (n=12). Patients assigned to ASM therapy underwent a thigh muscle biopsy to harvest 2–5 g of skeletal muscle, representing 10,000–50,000 cells. Isolated myoblasts were grown until as many as 600 million cells were available for transplantation. Patients in the ASM group received one of four doses − 30, 100, 300, or 600 million cells – delivered to the heart with a catheter guided by an investigational three-dimensional mapping system.
Nabil Dib, MD, University of California, San Diego, CA, and colleagues determined that all ASM transplant procedures were performed successfully and without injection-related complications, meeting the primary endpoints of feasibility and safety. At 12 months follow-up, there were no deaths, MI, or cerebrovascular events in either treatment group, although one patient in each treatment group was hospitalized for congestive heart failure.
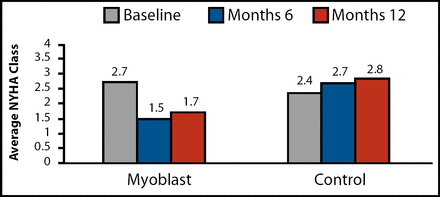
Functional capacity also improved in the transplantation group. Among ASM patients, mean NYHA class improved by roughly one class from baseline to 6 months (2.7 vs 1.5, respectively; p<0.0001) and 1 year (1.7; p<0.0001; Figure 1). Quality of life also appeared to improve in the ASM arm, as demonstrated by an improvement in Minnesota Living with Heart Failure Score from baseline to 6 months (p=0.02) and 1 year (p=0.002). By comparison, functional capacity and quality of life tended to worsen over time.
Average NYHA Class at 6 and 12 Months.
“Myocardial regeneration may play an important role in the treatment of heart attack and heart failure,” Dr. Dib said. Indeed, data from the CAuSMIC trial provide promising evidence to support the use of ASM transplantation in patients with MI-related heart muscle injury, he concluded. The US Food & Drug Administration has approved a phase 2, randomized, placebo-controlled trial in 165 patients to determine whether these findings can be replicated, Dr. Dib added.
Bone Marrow Stem Cells Improve Ejection Fraction
A second randomized trial demonstrated that IC injections of bone-marrow-derived cells (BMCs) improved left ventricular ejection fraction (LVEF) among patients with ST-elevation MI (STEMI) treated with thrombolytic therapy followed by PCI.
Based in Finland, the double-blind, placebo-controlled FINCELL trial enrolled 78 patients with acute STEMI. Patients were randomly assigned to treatment with IC injections of autologous BMCs or IC injections of placebo. Injections were administered 2–6 days after the index MI, immediately after implantation of paclitaxel-eluting stents.
At 6 months, patients in the BMC group had a significant increase in their global ejection fraction (EF) measured by angiography (from 58.8% to 65.9%; p=0.002), compared with no change in EF in the placebo group. Two-dimensional echocardiography also detected differences in LVEF—an increase of 4.0% in the BMC group and a decrease of 1.4% in placebo group (p=0.03).
No differences were observed in arrhythmia risk variables, including measures of heart rate variability, signal-averaged electrocardiogram, and prevalence of positive T-wave alternans tests. In addition, no differences in risk for restenosis, as measured by minimal lumen diameter and area of the stented lesion, were noted in the two treatment groups.
“Intracoronary BMC therapy is safe and has neutral effects on arrhythmia risk factors and restenosis of the stented target vessel,” lead study author Heikki Huikuri, MD, University of Oulu Hospital, Oulu, Finland, concluded.
Bone Marrow Stem Cells Do Not Improve Contractility
Another study of BMCs failed to show improvements in heart contractility following implantation, regardless of whether BMCs were delivered directly to the heart via intramuscular (IM) or IC injection. Findings of the IC/IM-BMC trial were presented by Keng-Leong Ang, MRCS, University of Leicester, United Kingdom.
A total of 62 patients scheduled to undergo elective coronary artery bypass grafting (CABG) were randomly assigned to one of three treatment groups: IC injection of BMCs (n=21), IM injection of BMCs (n=21), or no injection (n=20). The trial was designed to determine whether BMC treatment could improve the contractility of scarred heart tissue and to find which infusion method worked best.
Researchers found no differences in postoperative parameters of heart contractility among the three treatment groups. At 6 months, patients in all groups had similar parameters of wall motion assessment, systolic fractional thickening, end-diastolic volume, and end-systolic volume.
Although IM and IC administration of BMCs into scarred myocardium was safe, this technique did not improve systolic function of injected areas, did not reduce infarct size, and did not influence global LV function, Prof. Ang concluded.
- © 2007 MD Conference Express