Summary
More potent antiplatelet therapy with prasugrel reduced the rate of ischemic events compared with clopidogrel among patients with acute coronary syndromes with planned percutaneous coronary intervention (PCI). The TRITON-TIMI 38 Trial showed that patients treated with the novel thienopyridine prasugrel had a significant net clinical benefit compared with clopidogrel, the standard therapy for patients undergoing PCI.
- myocardial infarction clinical trials
- interventional techniques & devices
More potent antiplatelet therapy with prasugrel reduced the rate of ischemic events compared with clopidogrel among patients with acute coronary syndromes (ACS) with planned percutaneous coronary intervention (PCI). TRITON-TIMI 38 showed that patients treated with the novel thienopyridine prasugrel had a significant net clinical benefit compared with clopidogrel, the standard therapy for patients undergoing PCI.
“There was an early and sustained benefit that was apparent across the ACS spectrum,” said Elliott Antman, MD, Brigham and Women's Hospital, Boston, MA, who reported on the study. He added that the drug was associated with an increased risk of bleeding among specific subgroups of patients, indicating that the optimum use of the drug and its dosing are crucial issues to explore.
Dr. Antman explained that prasugrel produces rapid and high levels of platelet inhibition and that the trial was designed to test the hypothesis that a greater degree of platelet inhibition would reduce ischemic events as well as to evaluate the safety of prasugrel.
The multicenter trial enrolled 13,608 patients with ACS scheduled for PCI. Patients were randomly assigned to standard clopidogrel (300 mg loading dose and 75 mg daily for 6–15 months) or to prasugrel (60 mg loading dose and 10 mg daily for the same period).
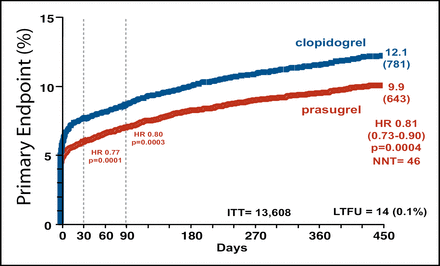
Prasugrel was associated with a 19% reduction in the primary endpoint, a composite of cardiovascular death, nonfatal myocardial infarction (MI), or stroke (9.9% for prasugrel vs 12.1% for clopidogrel; p=0.0004). The benefit of prasugrel was evident both early (3 days) and late (450 days) (Figure 1). Mortality was similar for the two groups. The “most dramatic effect,” said Dr. Antman, was a 52% reduction in stent thrombosis (1.1% for prasugrel vs 2.4% for clopidogrel; p<0.0001).
Cumulative Kaplan-Meier Estimates of the Rates of Key Study End Points During the Follow-Up Period.
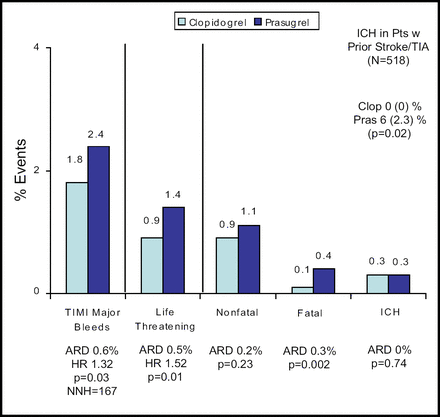
Significantly more TIMI major bleeding occurred with prasugrel (2.4%) than with clopidogrel (1.8%; p=0.03). Prasugrel use was also associated with significantly greater nonfatal, life-threatening, and fatal bleeding (Figure 2). The findings of a prespecified analysis of net clinical benefit significantly favored prasugrel (13.9% vs 12.2%; p=0.004).
Bleeding Events Safety Cohort (n=13,457).
The increased efficacy coupled with the increased risk for bleeding led the investigators to conduct post hoc exploratory analyses to identify subgroups of patients who did not have a net clinical benefit from prasugrel. These subgroups were found to be patients who were 75 years or older (HR, 0.99; p=0.92) or who weighed less than 60 kg (HR, 1.03; p=0.89). Patients with a history of cerebrovascular events also had no net clinical benefit as well as evidence of significant harm from prasugrel. Among patients with such a history, a composite endpoint of death from any cause, nonfatal MI, nonfatal stroke, or non-coronary artery bypass graft-related TIMI major bleeding was associated with a hazard ratio of 1.54 (p=0.04).
Further studies are needed to help define populations at increased risk for bleeding and determine optimal dosing. “Optimization of prasugrel maintenance dosing in a minority of patients may help improve the benefit-risk balance,” said Dr. Antman.
The findings of this study have been published: [Wiviott et al. NEJM 2007;357:2001–2015].
- © 2007 MD Conference Express