Summary
According to the updated results from the Swedish Coronary Angiography and Angioplasty Registry [SCAAR], which now contains up to 4 years of follow-up data for more than 60,000 patients, mortality rates in patients with drug-eluting stents are no higher than in those with bare metal stents.
- interventional techniques & devices clinical trials
According to the updated results from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR), which now contains up to 4 years of follow-up data for more than 60,000 patients, mortality rates in patients with drug-eluting stents (DES) are no higher than in those with bare metal stents (BMS).
The updated results, reported by Stefan James, MD, Uppsala Clinical Research Centre, Sweden, included the addition of 1 year of enrollment and follow-up data to the originally published report (Lagerqvist B et al. New Engl J Med 2007), which showed a higher mortality rate among the DES patients.
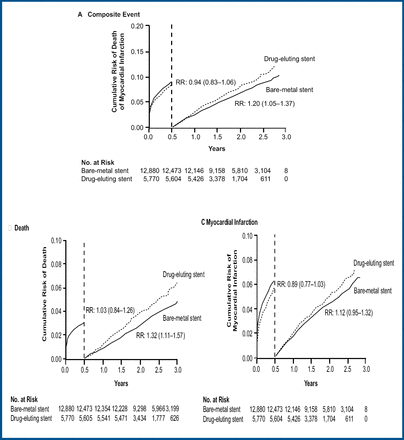
The latest data include outcomes from 35,266 patients who had undergone stent implantation (13,785 DES; 21,477 BMS) from January 1, 2003 – December 31, 2005 and for whom at least one year of follow-up was available. Patient characteristics are shown in Table 1.
Patient Characteristics
During the entire study period there were 4,160 MIs and 2,957 deaths. At 4 years, there was no difference in the primary endpoint of death/myocardial infarction (MI) (RR 1.01; 95% CI 0.94, 1.09), MI (RR 1.01; 95% CI 0.91, 1.11), or death alone (RR 1.03; 95% CI 0.94, 1.14) between patients treated with DES vs those who received BMS. However, treatment with DES was associated with significantly increased risk of MI (RR 1.25; 95% CI 1.09, 1.42) and death/MI (RR 1.17; 95% CI 1.06, 1.29) after the initial 6-month post-treatment. This late increase in risk was compensated by a lower event rate during the 6 months immediately after stent implantation, when significant reductions in the combined endpoint of death/MI (RR 0.85; 95% CI 0.77, 0.95) and in the rate of MI alone (RR 0.82; 95% CI 0.72–0.93) were observed (Figure 1).
Landmark Analyses – Total Cohort.
Copyright © 2007 Massachusetts Medical Society. All rights reserved.
The continuous low risk of late stent thrombosis of 0.5% annually in patients treated with DES was more than offset by a 3.5% absolute reduction in the risk of restenosis with DES compared to BMS.
“The results are very positive for the 8 million patients worldwide who have received DES and are concerned by the risk of death and adverse events,” said Prof. James. “But physicians should be concerned that we've not solved the problem of late stent thrombosis. They need to think carefully about patient selection.” According to Prof. James, patients with larger vessels, increased risk of bleeding and compliance issues should receive BMS, while those with bifurcations, lesions longer than 8mm, narrow vessels, and those with diabetes may benefit from DES.
This study is based on observational data and there are a number of factors that may have affected the results including increased awareness of the risk of DES and prolonged antiplatelet therapy, improved techniques utilizing higher balloon pressures and more accurate stent sizing, and improved stent design. Large prospective randomized trials of DES versus BMS that include different durations of dual antiplatelet therapy are needed.
- © 2007 MD Conference Express