Summary
This article discusses results of a pre-defined subgroup analysis from the bosentan for inoperable chronic thromboembolic pulmonary hypertension [BENEFiT] trial. The BENEFiT study is the first double-blind, placebo-controlled trial designed to evaluate the safety and efficacy of bosentan for patients with inoperable chronic thromboembolic pulmonary hypertension, one of the leading causes of pulmonary arterial hypertension.
- hypertensive disease
- thrombotic disorders clinical trials
Irene Lang, MD, Medical University of Vienna, Austria, presented the results of a pre-defined subgroup analysis from the bosentan for inoperable chronic thromboembolic pulmonary hypertension (BENEFiT) trial. The BENEFiT study is the first double-blind, placebo-controlled trial designed to evaluate the safety and efficacy of bosentan for patients with inoperable chronic thromboembolic pulmonary hypertension (CTEPH), one of the leading causes of pulmonary arterial hypertension (PAH).
CTEPH is characterized by obstruction of pulmonary vessels with organized thromboemboli. The obstruction promotes increased pulmonary vascular resistance, progressive PAH, and, subsequently, right heart failure. The treatment of choice for CTEPH is the surgical removal of the thrombus in the major pulmonary vessels (pulmonary endarterectomy). However, as many as 50% of patients may not be candidates for this surgery [Nick H & Kim S. AmThorac Soc 2006], and among patients who do undergo pulmonary endarterectomy, PAH persists or recurs in 10–20% [Dartevelle P et al. Eur Respir J 2004]. Thus, other treatment options are needed. Prof. Lang and her colleagues chose to evaluate the use of bosentan in this setting because of its vasodilatory effects, which would reduce pulmonary vascular resistance.
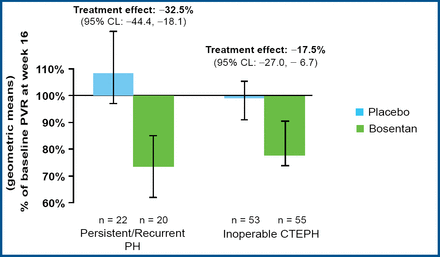
The BENEFIT trial involved 157 patients who were randomly assigned to receive bosentan (n=77) or placebo (n=80). The initial dose of bosentan was 62.5 mg BID for 4 week s, followed by 125 mg BID for 12 week s. The primary endpoints were pulmonary vascular resistance and 6-minute walk distance. Secondary endpoints included cardiac index, the level of N-terminal brain natriuretic propeptide (NT-pro-BNP), and the Borg dyspnea index (a measure of breathlessness). Subgroup analysis was performed to compare the treatment effects of bosentan (at 16 weeks) for the 113 patients who were not candidates for surgery (55 patients in the bosentan group; 58 in the placebo group) with the effects for the 44 patients who had persistent or recurrent PAH after pulmonary endarterectomy (22 patients in each group).
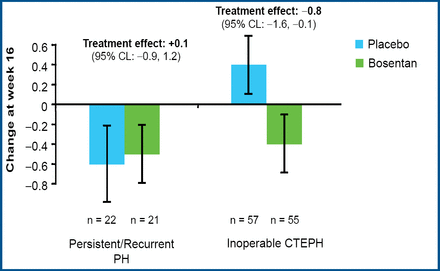
Prof. Lang reported that treatment with bosentan produced clinically relevant improvement in cardiac hemodynamic characteristics. Bosentan significantly reduced the pulmonary vascular resistance in both groups (p<0.0001), with a 17.5% reduction for inoperable patients and a 32.5% reduction for patients with persistent or recurrent PAH after pulmonary endarterectomy (Figure 1). Bosentan also significantly increased the cardiac index (mean 0.31 vs 0.25; p=0.0007) and significantly decreased the level of NT-pro-BNP (mean −654 vs −526; p<0.05) compared with placebo. Treatment with bosentan led to improvement in the Borg dyspnea index in patients with inoperable CTEPH but not in patients who underwent surgery (Figure 2).
Bosentan Reduced PVR.
Borg Dyspnea Index Improved in Patients with Inoperable CTEPH.
Bosentan did not improve the 6-minute walk distance, the other primary endpoint of the study, in either group (−11.5 meters in patients with persistent/recurrent PAH and 8.8 meters in inoperable patients; p=0.5449). Prof. Lang suggested that this is most likely because the 16-week time point was not long enough for a difference in exercise capacity to occur. She added, “These are also older patients [mean age, 63 years] with many comorbidities, perhaps further diluting any change in the 6-minute walk test.” The safety of bosentan was consistent with that found in other studies of the drug for PAH.
In concluding, Prof. Lang noted, “Prognosis in CTEPH patients is highly related to hemodynamics and hemodynamics is a limitation to surgery. So, we may be benefiting patients long-term or, in some cases, actually getting inoperable patients improved hemodynamically to where they can undergo surgery.” She added, “The results suggest we do have a medical option now for a no-option population.”
- © 2007 MD Conference Express