Summary
This article gives brief history of the advancement of cytokine based therapy and discusses the role of these agents in the future.
- rheumatological autoimmune disorders
- inflammatory disorders
Jean-Michel Dayer, MD, University Hospital, Faculty of Medicine, Geneva, presented the Fred Wyss lecture at the 2007 EULAR congress in Barcelona. He gave a brief history of the advancement of cytokine based therapy and discussed the role of these agents in the future.
The development of what Prof. Dayer referred to as the “cytokine community” has followed a repeatable sequence. At first, an individual cytokine is identified and associated with a particular function. Later, it is found to have many other functions. At some point, the cytokine is cloned with the assumption that there will be one function associated with one factor, but the result is often that new, unexpected members of the cytokine family are identified and novel functions are revealed. This, in turn, leads to both clinical and biological surprises, species problems, side effects, and antagonists and agonists within the same family. The TNF, IL-1, IL-6 families are excellent examples of this process.
TNF was discovered many years ago as a serum factor that causes necrosis of tumors in mice. Years later, the TNF receptor was shown to be expressed by mammalian cells. This led to the discovery of a superfamily of transmembrane proteins and the identification of gene families that include 18 ligands and 28 receptors.
TNF has been found to induce collagenase and PGE2 in human synovial cells from patients with RA [Dayer J-M et al. J Exp Med 1985] and to induce bone resorption [Saklatvala J et al. Nature 1986]. It was determined to be involved in collagen-induced arthritis [William RO et al. Proc Natl Acad Sci 1982] and was detected in the biological fluids of rheumatoid arthritis (RA) patients [Saxne T et al. Arthritis and Rheum 1988]. A key observation was that TNF is very important in the hierarchy of the cytokines, since antibodies to TNF decrease other downstream cytokines [Brennan FM et al. Lancet, 1989]. Using a chimeric monoclonal antibody to TNF-α as a treatment for RA leads to impressive clinical results [Elliott JM et al. Arthritis Rheum 1993].
The developmental history of cytokines and IL-1 followed a path similar to the TNF developments. Many functions were attributed to IL-1, such aslymphocyte activating factor (LAF) osteaoclast-activating factors (OAF), endogenous pyrogen (EP) and related to RA mononuclear cell factor (MCF) inducing collagenase and PGE2 [Dayer et al. Science 1977]. IL-1 biology still leads to some surprises. For example, in a mouse model, local hippocampal over expression of IL-1 beta in an Alzheimer's diseased transgenic mouse resulted not in the expected exacerbation of the amyloid beta plaque deposition common in Alzheimer's disease, but instead in plaque amelioration [Solomon S et al. J Clin Invest 2007]. IL-1 receptor antagonists (IL-1Ra) was shown to inhibit insulin production in cultured rat pancreatic islets [Dayer-Métroz MD et al. J of Autoimmunity 1989]. Of great interest was the finding that the expression of the IL-1Ra was reduced in the pancreatic islets of patients with type 2 diabetes mellitus. The blockade of IL-1 with anakinra improved glycemia and beta-cell secretory function and reduced markers of systemic inflammation [Larsen CM et al. N Eng J Med 2007].
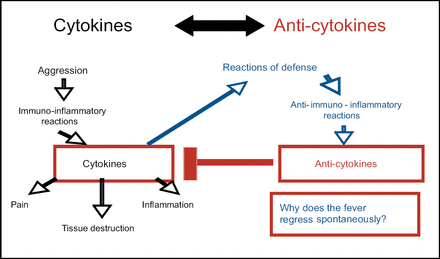
Prof. Dayer started his EULAR lecture by posing the question “Why does a patient's fever regress spontaneously?” He then proposed the following hypothesis: An insult to the system causes an immuno-inflammatory reaction; cytokines are released that cause pain, tissue destruction, and inflammation. A defensive anti-immuno-inflammatory reaction occurs, including the release of anti-cytokines that block the inflammation caused by the originally released cytokines (Figure 1), thus causing the fever to regress.
Spontaneous Fever Regression.
Proof of this can be found in a study conducted by Prieur et al, in which IL-1 activity and inhibition were studied in serum and urine from 9 patients with systemic juvenile chronic arthritis (S-JCA) [Prieur A et al. Lancet 1987]. In afebrile patients, IL-1 bio-activity was normal or high. Serum from 2 afebrile S-JCA patients taken during a period of severe disease activity had an enhancing effect on the bio-activity of exogenous IL-1. In contrast, febrile patients' serum and urine IL-1 bio-activity was low, apparently reflecting the presence of a strong inhibitor of IL-1 activity measured by the inhibition of prostaglandin E2 production by synovial cells. This inhibition was greatest at the time of peak temperature, suggesting the possibility of feedback regulation during fever. Recently, anakinra has been shown to be successful in treating patients with adult-onset Still's disease [Fitzgerald JD et al. Arthritis Rheum 2005; Vasques G et al. Ann Rheum Dis 2005] and, surprisingly, in acute gout [So A et al. Arthritis Res Ther 2007].
Where are the initial events in RA? Locating the site of disease-initiating events is still under debate. Is it at the systemic level (extra-articular site), or in the bone marrow, or locally (articular site)? Support for systemic localization comes from Binstadt and colleagues [Nat Immunol 2006]. Using observations in the K/BxN murine arthritis model, they uncovered novel pathways underlying the site-specific localization of inflammation driven by immune complexes and triggered by sensitization to non-specific Ag at an extra-articular level. Such a hypothesis has been reviewed by Pitzalis C et al. [Trend in Immunology 2006].
Within both the bone marrow and the synovium, fibroblastic stromal cells play an important role in supporting the differentiation and survival of normal cells. They also contribute to the pathologic processes. A possible argument for the localization of the initiating event in the bone marrow could be the presence of nurse cells within the synovium that foster inflammation. These nurse cells may contribute to the localization of inflammation within specific joints. It has also been noted that fibroblastic stromal cells from epiphyseal bone marrow can migrate into the joint space, forming synovial tissue in collagen-induced arthritis [Ochi T et al. Arthritis Res Ther 2007].
What about the local articular site? The overgrowth of synovial tissues is critical in the pathogenesis of rheumatoid arthritis (RA). The expression of Synoviolin (SYN), an E3 ubiquitin ligase, is upregulated in arthritic synovial fibroblasts and is involved in the overgrowth of synovial cells during RA. The proinflammatory cytokines IL-1β and TNFα induce the overgrowth of synovial cells by upregulating SYN expression via the Erk1/-ETS1 pathway [Gao B et al. Arthritis Res Ther 2006]. Another molecule, Cadherin-11, strongly determines the behavior of synovial cells in their proinflammatory and destructive tissue response in inflammatory arthritis [Lee DM. Science 2007].
Prof. Dayer also spoke briefly about the new research affecting cytokine understanding, such as cell to cell contact between T lymphocytes and monocytes for IL-1/TNF production and the environmental influence of Apolipoprotein A1-HDL blocking [Hyka N. Blood 2001; Dayer JM et al. Autoimmune Res 2004]; on the production of cytokines, and the role of adipose tissue.
Adipocytokines are cytokines secreted by adipose tissue, the source of production and site of action of several pro- and anti-inflammatory cytokines. White adipose tissues are the major producer of the anti-inflammatory IL-1Ra [Juqe-Aubry CE et al. J Clin End Metabol 2004]. Some adipocytokines such as adiponectin and leptin affect immune and inflammatory functions. A new proinflammatory adipocytokine (Visfatin) has recently been identified as an adipocytokine that activates human leukocytes and induces cytokine production [Moschen AR et al. J Immunol 2007].
Emerging cytokine targets in RA, eg, IL-6, IL-15, and IL-32, are in multiple stages of development. New cell therapies using T-regulatory cells, hematopoietic stem cell transplantation, and mesenchymal stem cells are also in development.
New treatments based upon vaccination with cytokines are emerging. An anti-cytokine induction of autoimmune protection against both acute and chronic hTNFα exposure has been demonstrated. Thus, an effective and safe vaccination against a human cytokine may be achievable [Le Buanex H et al. Proc Natl Acad Sci USA 2006].
A number of peptide, and peptidomimetic-based approaches (such as TCR-peptide vaccines and peptides derived from heat shock protein), and antisense oligonucleotide are currently being tested in animal models to treat inflammatory arthritis.
“We have learned, and will continue to learn, a great deal about cytokines, a remarkable class of potential disease altering agents that will play a major role in future therapeutics, and lead to more ‘ad personam’ treatment depending upon the subtypes of diseases and the gene status.” Prof. Dayer concluded.
- © 2007 MD Conference Express