Summary
This article reviews a phase 3 trial of biologically-based therapy reduction for intermediate risk neuroblastoma in young patients. Also discussed is a final analysis of the Head Start I and II protocols, which looked at outcome sof children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy.
- Head & Neck Cancers
Neuroblastomas account for 15% of cancer-related deaths occurring in childhood. The children's cancer group study (CCG) 3881 showed that the chances of overall survival (OS) were greater than 80% with aggressive chemotherapy and surgery. While these results are encouraging, the burden of treatment is quite severe in these young patients. The primary goal of this trial (COG 3961) was to achieve a 3-year OS >90% for intermediate risk (IR) neuroblastoma with reduced therapy and hopefully toxicity compared to historical controls.
Treatment was administered on an outpatient basis using the most active agents available for neuroblastoma. Standard therapies were modified, however, with the aim of reducing toxicity: cisplatin was replaced with carboplatin and the total dose of anthracyclines were limited to a cumulative dose of below 100mg/m2. Patients were stratified according to favorable or unfavorable biology; treatment was administered over 64 days in the favorable group with 4 cycles, and over 168 days in the unfavorable group with 8 cycles.
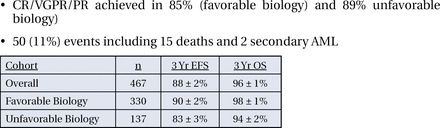
Between March of 1997 and May of 2005, 463 eligible patients were enrolled in the study; roughly 70% had favorable biology and 30% had unfavorable biology. Fifty-six (56) percent of the patients had stage III disease (105 children and 156 infants), 37% were infants with stage IV disease and 7% were infants with stage IV-S disease. The study was designed such that patients with favorable biology were to receive 4 cycles of treatment, but if they did not achieve a response during this time, they were then eligible to receive all 8 cycles; 40% of patients in the favorable biology group did so.
Again, one of the goals of this study was to reduce overall toxicity. Reversible hematological toxicities were seen in 69% of the patients. Importantly, however, renal, cardiac and hearing toxicities were observed in less than 2% of the patients, if transient grade 3/4 toxicities in blood pressure were excluded. Non-hematological toxicities were significantly less in course two than in course one, which is almost certainly related to the release of disease burden by the end of course one. Finally, deaths due to infection were low at 0.8%.
Response rates (comprised of complete response, very good partial response, and partial response) were achieved in 85% of patients in the favorable biology group and 89% of the unfavorable biology group. In both groups, 3-year OS was achieved in over 90% of the patients (Figure 1).
3-Year Event Free Survival and Overall Survival in the Favorable Biology Group, the Unfavorable Biology Group and in Both Groups Combined.
The primary hypothesis of this study, therefore, was confirmed, with 3-year OS >90% for intermediate risk neuroblastoma. More importantly, compared with the historical control CCG 3881, this outcome has been achieved with a very significant reduction in therapy (Table 1). Future phase 3 trials will prescribe duration of therapy, at least in part, in accordance with the results of this trial.
Overall Survival and Treatment Burden in CCG 3881 and COG A391.
Outcome of Children Less Than Three Years Old at Diagnosis with Non-Metastatic Medulloblastoma Treated with Chemotherapy on the “Head Start” I and II Protocols: Final Report
The majority of malignant brain tumors in children develop within the first 6 years of life. The most common type is medulloblastoma (MB), an aggressive tumor which grows rapidly, recurs often and is susceptible to metastasis. The standard of care for older children with medulloblastoma is radiation therapy, followed by chemotherapy. However, this treatment is often associated with several adverse effects, including learning and attention difficulties, short-term memory deficits, social adjustment problems, hearing, speech and language problems, and impaired physical growth. Therefore, a strategy to treat this type of disease without radiation is evident; the goals of Head Start I and II were to address this issue.
The treatment plan was the same in both protocols. After maximum surgical resection, patients underwent 5 cycles of induction chemotherapy, followed by an extensive disease evaluation. If the analysis showed residual tumor, the investigators encouraged a re-resection; if the patients progressed by this time, they were removed from the study. Patients who were either stable at the end of induction, or had responsive disease, underwent consolidation chemotherapy with autologous hematopoietic stem cell rescue. Finally, patients with no evidence of disease after these treatments (the majority of patients in this trial) did not receive radiation.
Twelve (12) patients were treated in Head Start I and 9 patients were treated in Head Start II. The male and female ratios were similar in both studies, though patients tended to be younger in Head Start I. Gross total resection was more successful in Head Start II. Three (3) patients in Head Start I and 6 patients in Head Start II were desmoplastic. Event-free survival (EFS) for all patients in the cohort was 52% at 5 years, while the overall survival (OS) was 70% at 5 years.
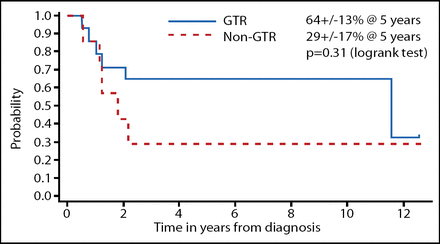
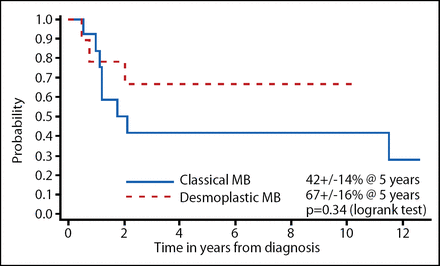
The investigators dissected the data according to the prognostic factors. The EFS when classified by extent of resection is shown in Figure 1 and desmoplastic vs classical MB is shown in Figure 2. Although these results were not statistically significant, a gross total resection and desmoplastic MB did trend toward improved EFS; OS did not show this same trend. The total radiotherapy-free survival among the surviving patients in this study was 73%.
EFS by Extent of Resection in Head Start I and II.
EFS Characterized by Desmoplastic or Classical MB in Head Start I and II.
There were 3 toxic deaths in Head Start I and 1 in Head Start II. Five (5) patients relapsed in Head Start I, whereas only 2 patients did so in Head Start II; all relapses developed within 25 months and the majority occurred locally. Overall quality of life (QoL) was within the average range at both times of assessment (70 months and 124 months); younger patients displayed fewer behavioral problems and higher adaptive function at both time points.
In conclusion, EFS in this study mirrors those reported in the German HIT study [Rutkowski et al. New Eng J Med 2005]. Incomplete resection and absence of desmoplasia trend toward inferior EFS, but not OS. These results show that, despite administering the myeloablative chemotherapy, these patients can be retrieved with either more chemotherapy or irradiation. Although the number of patients is limited in these studies, the QoL was within the normal range in the Head Start I study and it was improved in 3 out of 4 patients in the Head Start II study.
Photo Courtesy © ASCO/Todd Buchanan 2007
- © 2007 MD Conference Express