Summary
This article discusses a phase 3 Study of vinflunine Versus docetaxel in patients with advanced non-small cell lung cancer (NSCLC) previously treated with a platinum-containing regimen, as well as results from the IRIS Study, and the use of surgery alone or surgery plus induction paclitaxel/carboplatin chemotherapy in early stage NSCLC.
- Respiratory Cancers
- Cancer
Second-line treatment in patients with non small cell lung cancer (NSCLC) is challenging because the number of patients which are active is very limited, the response rate is well below 10%, and the prolongation of life is within weeks to months, rather than months to years. Additionally, there are very few drug options for treatment in the second-line setting; the three FDA-approved drugs are docetaxel, pemetrexed and erlotinib. Docetaxel has long been the standard for use in this setting; pemetrexed was approved based on the surrogate endpoint of response rate, though overall survival benefits have yet to be determined. Erlotinib has been shown to produce survival benefits when used in a second-line or even third-line regimen, though additional options for these patients are still necessary. The main objective of this study was to confirm non-inferiority of possible new second-line therapy, vinflunine (VFL), docetaxel (DTX), with respect to progression-free survival (PFS).
Patient characteristics were well balanced between the two arms of the study and were very typical for this category of patients; the majority of patients were male, the tumor extent at entry was mainly metastatic and the majority of patients had more than one organ involved. All patients received platinum-based chemotherapy.
The extent of toxicity is very important in second-line treatment strategies as the patients have already been exposed to cytotoxic drugs. The number of cycles tolerated between the study arms was very comparable: 3.5 vs 3.7 in the VFL and DTX arms, respectively.
Neutropenia was the most frequent adverse event at 39%, though there was no significant difference between the two study arms (Table 1). The only significant difference observed was in grade 3 and 4 anemia; there was a greater incidence of anemia associated with VFL administration. The incidence of the non-hematological toxicities alopecia, nail disorders, edema, diarrhea and myalgia were significantly more in the DTX arm. Conversely, injection site reactions, vomiting, abdominal pain and constipation were much more frequent in the VFL arm. The lower incidence of vomiting in the DTX arm may be due to the administration of corticosteroids for 3 days prior to chemotherapy.
Haematological Toxicity Observed in Patients Receiving VFL and DTX.
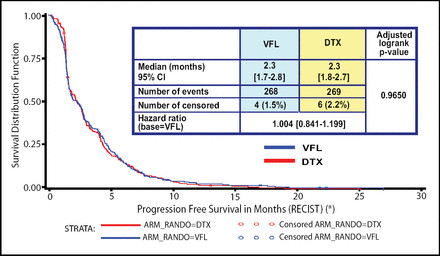
There were no significant differences in objective response rates or in disease control between the two study arms. Additionally, there were no differences in the primary objective of PFS (Figure 1); the median PFS was 2.3 months for both agents. Almost identical overall survival rates were likewise observed.
PFS Between Patients Receiving VFL and DTX.
“In conclusion, there is no clear winner in this trial,” commented Maciej Krzakowski, MD, Sklodowska-Curie Memorial Cancer Centre & Institute of Oncology, when reflecting on the results of this phase 3 trial.
Both VFL and DTX show similar efficacy in patients treated with a platinum containing regimen for advanced NSCLC patients. Different toxicities were observed between the two drugs, but both were manageable allowing a median relative dose intensity of 98%. Vinflunine may therefore offer a new and useful alternative for second-line therapy in advanced NSCLC.
Surgery Alone or Surgery Plus Induction Paclitaxel/Carboplatin Chemotherapy in Early Stage Non-Small Cell Lung Cancer
Non-small cell lung cancer (NSCLC) patients with clinically staged IB-IIIA disease have very poor 5-year survival rates. A large phase 3 trial found that preoperative paclitaxel/carboplatin was feasible and encouraged survival [Pisters K. JTCVS 2000]. Therefore, the main objective of this study was to determine if three cycles of preoperative paclitaxel/carboplatin chemotherapy improves survival when compared to surgery alone in stage IB, II and IIIA NSCLC. Additionally, the investigators compared operative mortality, toxicities and response rates among the trial arms. It should be noted that at the time of trial design, phase 3 studies supported preoperative, but not adjuvant chemotherapy.
The targeted accrual number for this study was 600 patients, but accrual was stopped prematurely due to developing data indicating that adjuvant chemotherapy was beneficial. Therefore, only 354 patients were included in this trial; 18 were ineligible. Eligible patients were randomized to receive either 3 cycles of paclitaxel (225mg/m2 as a 3 hour infusion)/carboplatin (AUC=6) followed by surgical resection or surgery alone. Patient characteristics were balanced between the two arms: the average age was 64–65 years, roughly one-third of the patients were female, about two-thirds of the patients had stage IB-IIA disease and squamous cell histology predominated with 34% and 42% in the preoperative and surgery alone arms, respectively.
Induction Chemotherapy Results
A total of 79% of patients completed all three cycles of induction therapy; adverse events and refusal were the most common causes of non-compliance. Three percent (3%) of patients had a complete response to therapy, 38% of patients had a partial response, 43% of patients had stable disease and 9% of patients had progressive disease; the remainder of patients could not be assessed. Additionally, induction chemotherapy was well tolerated; grade 3 and 4 events were uncommon, with the exception of neutropenia which was seen in 48% of patients.
Surgery Results
Complete resection was performed in 94% of the preoperative chemotherapy group and 89% of the surgery alone arm (p=0.10). Seven (7) out of 169 patients in the postoperative period in the chemotherapy arm died, while 4 out of 167 patients died in the surgery alone arm.
Survival Results
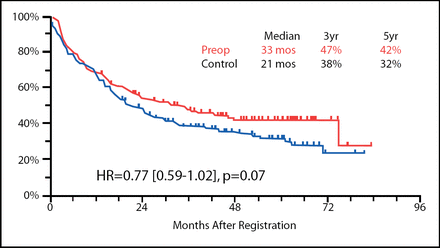
Progression-free survival (PFS) was not statistically different between the two arms of this trial at a median follow-up time of 53 months (Figure 1), though the results tended toward statistical significance favoring preoperative chemotherapy (33 months vs 21 months in the preoperative and surgery alone arms, respectively; p=0.07). The overall survival rates, however, did not show this trend.
PFS in Patients Receiving Preoperative Chemotherapy vs Those Who Underwent Surgery Alone.
Although the results of this trial did not achieve statistical significance, they continue to support the role of chemotherapy in patients with operable NSCLC. The authors point out that the hazard ratios achieved in this study are similar to those of other trials that did reach statistical significance. Additionally, this trial was stopped early due to positive results in trials using adjuvant chemotherapy, thus trials comparing preoperative and postoperative chemotherapy are warranted. Finally, an individual patient-based meta-analysis of induction chemotherapy is being conducted and will hopefully elucidate the future role of such treatment strategies.
Results from the IRIS-Study
Small cell lung cancer (SCLC) comprises approximately 15–20% of all lung cancers, and in the majority of patients, is diagnosed in the extensive disease (ED) stage and has relatively poor prognoses. Indeed, with currently available therapies, the median survival time is only between 7–10 months. Recent studies have shown conflicting activities of irinotecan, a topoisomerase I inhibitor, in the treatment of SCLC [Noda K. N Eng J Med 2000; Hanna N. J Clin Onc 2006]. The purpose of this study was to determine if the superiority of irinotecan over etoposide, as demonstrated in the study by Noda et al, could be replicated.
The patient characteristics were well balanced between the two study arms; of note, a large number of patients were >70 years of age and almost 50% of patients were in performance status (PS) category 2 or higher. After stratification for PS, age and institution, patients were randomized to receive carboplatin and either irinotecan intravenously on day 1 and every 21 days thereafter (IC), or etoposide orally on days 1–5 and repeated every 21 days (EC). There were no limitations in this study of WHO PS; brain metastases were allowed, though unimpaired mental status was required. Patients with previous systemic cytotoxicity and other active cancers were, however, excluded. The primary endpoint for this study was overall survival (OS) and the secondary endpoints were quality of life (QoL) and complete response rate (CR).
Approximately 90% of patients received at least 2 cycles of chemotherapy, with the full 4 cycles being administered to 80% of patients. Patients receiving IC had a significant survival advantage over those receiving EC (8.5 months vs 7.1 months, respectively; p=0.02). Additionally, 18 patients receiving IC were classified as complete responders, whereas only 7 receiving EC were classified as such (p=0.02). There were no significant differences between grade 3 and 4 toxicities for leucopenia or anemia; thrombocytopenia was significantly more common in the EC group, while diarrhea was more frequent in the IC arm (Table 1).
Toxicity Profile of Patients Receiving IC vs EC Treatment.
The results of this study support the use of carboplatin plus irinotecan in the treatment of extensive disease SCLC. This drug regimen yields a significantly longer median OS and does not compromise quality of life. While the median survival time was shorter than those observed in similar trials, the investigators believe this was the result of the selection of a very representative patient population. In other words, no upper age limit or PS requirement was defined for this study which likely resulted in a sicker population of patients at onset. While the toxicities varied depending on the study, overall QoL was not severely different in the two study arms. Finally, this study supports the data published by Noda et al in 2000 and adds to the growing body of data defining irinotecan as a potent SCLC drug.
- © 2007 MD Conference Express