Summary
This article discusses multiple studies using capecitabine plus oxaliplatin (XELOX), 5-fluorouracil (5-FU), leucovorin, and oxaliplatin (FOLFOX-4), and Infusional 5-FU/LV (FOLFOX-6), as well as the the MOSAIC and XELOX-1 Trials for the treatment of metastatic colorectal cancer.
- Gastrointestinal Cancers
NO16999 (XELOX-1) was an open-label randomized trial designed to demonstrate the non-inferiority of XELOX and FOLFOX-4 in patients with previously untreated metastatic colorectal cancer. The primary endpoint was the non-inferiority of progression-free survival (PFS) following treatment with XELOX vs FOLFOX-4.
After pivotal phase 3 data for bevacizumab became available [Hurwitz H et al. NEJM 2004], the XELOX-1 protocol was amended for all newly enrolled patients. In a blinded second randomization, patients received additional treatment with bevacizumab or placebo. A second primary endpoint was added: PFS of bevacizumab vs placebo in patients treated with chemotherapy.
The research group previously reported non-inferiority in terms of PFS of XELOX vs FOLFOX4 for the whole study population. [Cassidy J et al. Ann Oncol 2006] Two new reports presented at ASCO provided an update on the roles of bevacizumab and type of chemotherapy, respectively, on long-term outcome.
The study presented by Leonard Saltz, MD, Memorial Sloan-Kettering Cancer Center, New York, focused on progression-free and overall survival (OS) in patients enrolled after the protocol amendment. This cohort included patients treated with FOLFOX-4 plus placebo (n=351), FOLFOX-4 plus bevacizumab (n=349), XELOX plus placebo (n=350), and XELOX plus bevacizumab (n=350). The median age of patients in the FOLFOX-4 and XELOX arms was 60 and 61 years, respectively. Approximately 25% of patients had received adjuvant chemotherapy prior to enrolling in the XELOX-1 trial.
The addition of bevacizumab to oxaliplatin-based chemotherapy significantly improved PFS (HR, 0.83; p=0.0023). The median PFS in the bevacizumab and placebo groups was 10.4 months and 7.9 months, respectively (p<0.0001). Time to treatment failure was 6.9 months and 6.0 months, respectively (p=0.0030). The addition of bevacizumab was associated with a modest survival benefit as well. Median OS was 21.3 months for bevacizumab-treated patients and 19.9 months for those in the placebo group (p=0.0769).
Patients in the placebo group were more likely to discontinue therapy due to disease progression events. Progression was the reason for treatment discontinuation in 56% of placebo recipients compared with 38% of bevacizumab recipients. In contrast, a higher proportion of patients discontinued therapy because of adverse events in the bevacizumab arm (31%) compared with the placebo arm (21%). Most treatment discontinuations were associated with chemotherapy-related toxicities rather than bevacizumab-related events.
The majority of patients in the placebo (75%) and bevacizumab (80%) arms experienced at least one grade 3/4 adverse event. Less than 1% of patients in either group experienced GI perforations.
The second analysis presented by lead author Jim Cassidy, MD, Glasgow University, Scotland, focused on PFS and OS among patients treated with XELOX or FOLFOX-4. In this analysis, data from all patients treated with XELOX (with or without bevacizumab) were pooled and compared to data from all patients who received FOLFOX-4 (with or without bevacizumab).
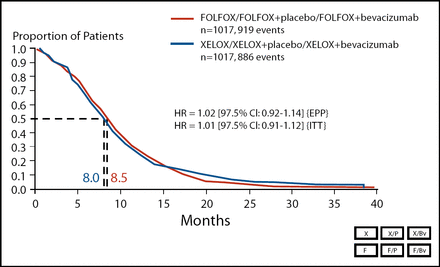
The co-primary endpoint of the XELOX-1 study – the non-inferiority of XELOX vs FOLFOX-4 for PFS – was met. Median PFS was 8.0 months in the XELOX group (n=1,017) and 8.5 months in the FOLFOX-4 group (n=1,017; Figure 1). OS was equivalent in the two treatment arms. In the XELOX group, median OS was 19.8 months, compared with 19.6 months in the FOLFOX-4 group.
Primary Endpoint PFS (ITT Population).
In addition to conducting a pooled analysis, Cassidy and colleagues evaluated XELOX vs FOLFOX-4 only in patients who received bevacizumab in addition to chemotherapy. In this analysis, the median OS was 21.4 months in the XELOX/bevacizumab group (n=349) and 21.2 months in the FOLFOX-4 group (n=350).
“The rationale for this study was to replace a complex intravenous-based regimen with a simple oral-based regimen,” explained Dr. Cassidy. “That's what XELOX does. You don't have a dent in the activity [compared with FOLFOX-4], and it's a lot easier for patients.”
“The key issue is quality of life. Instead of being in my ward, you can be at home,” Dr. Cassidy continued. “You see me less; you see your family more. This seems like a good idea to me.”
In a third analysis, the research group assessed survival in patients who enrolled in the trial prior to the protocol amendment. These patients were treated with chemotherapy only, and did not have an additional randomization to bevacizumab or placebo. In this group, the median OS was 18.8 months in XELOX recipients (n=317) and 17.7 months in FOLFOX-4 recipients (n=317).
The safety profiles of XELOX and FOLFOX-4 were balanced in this updated analysis. A similar proportion of patients in the XELOX group (26%) and FOLFOX-4 group (24%) discontinued therapy due to adverse events.
Efficacy and Safety Findings from a Randomized Phase 3 Study of Capecitabine plus Oxaliplatin (XELOX) vs Infusional 5-FU/LV (FOLFOX-6) for Metastatic Colorectal Cancer
This prospective, randomized, phase 3 trial was designed to demonstrate the non-inferiority of XELOX vs the FOLFOX-6 regimen as first-line treatment in metastatic colorectal cancer.
XELOX is a combination of capecitabine (1,000 mg/m2 twice daily days 1–14) and oxaliplatin (130 mg/m2 day 1). XELOX is administered every 3 weeks for a maximum of 8 cycles. FOLFOX-6 is a combination of oxaliplatin (100 mmg/m2 day 1), LV (400 mg/m2 over a 2-hour infusion on day 1), and 5-FU (400 mg/m2 intravenous bolus followed by 2,400–3,000 mg/m2 over a 46-hour infusion on day 1). FOLFOX-6 is administered every 2 weeks for a maximum of 12 cycles.
A total of 306 patients were randomly assigned treatment to XELOX (n=156) or FOLFOX-6 (n=150) for 6 months and followed for a median of 18.8 months after randomization. The primary endpoint was the non-inferiority of XELOX vs FOLFOX-6 for best response based on RECIST criteria. Patients were evaluated for overall response (OR), a combination of complete response (CR) and partial response (PR). Secondary endpoints included PFS, OS, and safety.
Patients in the XELOX group received a mean of 6 treatment cycles (range, 0–8), compared with 9 mean treatment cycles (range, 0–12) in the FOLFOX-6 group. The median relative dose intensities of oxaliplatin were 99.6% and 95.4% in the XELOX and FOLFOX-6 groups, respectively. Among XELOX recipients, the mean relative dose intensity for capecitabine was 100%. For patients treated with FOLFOX-6, the mean relative dose intensity of infusional 5-FU was 85.0%.
The primary outcome of this trial was achieved; XELOX was shown to be non-inferior to FOLFOX-6 in this patient population, though there were slight numerical advantages to the FOLFOX-6 regimen. The overall response rate was 42% in the XELOX group (including 2% CR and 40% PR) and 46% in the FOLFOX-6 group (including 0.5% CR and 45.5% PR). Disease control rates – a combination of CR, PR, and stable disease – were 84% in the XELOX arm and 87% in the FOLFOX-6 arm.
“These data show that it is possible to replace infusional 5-FU, which does not have a convenient method of administration, with capecitabine, an oral agent,” said lead study author Dr. Michel Ducreux, MD, Gustave Roussy Institute, Villejuif, France. “This approach is much more convenient for patients,” he said.
In addition to treatment response, long-term outcomes were also similar in the two treatment groups. Median PFS was 8.8 months in the XELOX group and 9.3 months in the FOLFOX-6 group. Median OS was 19.9 months and 20.5 months in the XELOX and FOLFOX-6 arms, respectively.
Patients in the XELOX and FOLFOX-6 groups reported generally similar adverse events. Among serious adverse events, a higher proportion of patients in the XELOX arm reported grade 3–4 hand-foot syndrome (3%, vs 1% in the FOLFOX-6 arm). In contrast, more patients in the FOLFOX-4 arm than in the XELOX arm experienced grade 3–4 nausea (6% vs 3%; p=ns), asthenia (9% vs 8%; p=ns), alopecia (4% vs 2%; p=ns), neutropenia (47% vs 5%; p<0.05), febrile neutropenia (6% vs 0%; p<0.05), and neuropathy (25% vs 11%; p<0.05).
Phase 3 Trial of Capecitabine plus Oxaliplatin (XELOX) vs 5-fluorouracil (5-FU), Leucovorin, and Oxaliplatin (FOLFOX4) as Second-Line Treatment for Patients with Metastatic Colorectal Cancer
This randomized phase 3 trial was designed to establish the non-inferiority of XELOX compared with FOLFOX-4 in patients with metastatic colorectal cancer who had failed first-line therapy with an irinotecan-based regimen.
Patients were randomly assigned to treatment with XELOX (oxaliplatin 130mg/m2 iv, capecitabine 1,000mg/m2 bid oral × 14 days, every three weeks) or FOLFOX-4 (as described previously). The primary endpoint was the non-inferiority of XELOX compared with FOLFOX-4 with regard to progression-free survival (PFS). Secondary endpoints included overall survival (OS) and safety.
Baseline characteristics were well balanced in the XELOX (n=314) and FOLFOX-4 (n=313) treatment groups. The median age of patients was 61 and 60 years, respectively, and 62% and 61% of patients were male. The distribution of baseline ECOG PS scores was similar (46–48% PS 0; 44–47% PS 1; and 7–8% PS 2). Primary tumor site, number of metastatic sites, and prior treatment history was also comparable in the XELOX and FOLFOX-4 arms.
Adherence to treatment schedules was similar in the XELOX and FOLFOX-4 arms. Patients in the XELOX group received a median of 6 treatment cycles over a median of 3.9 months. Those in the FOLFOX-4 arm received a median of 8.5 cycles over a median of 3.9 months. Approximately one-third of patients in each treatment group received 24 weeks of treatment. In the XELOX arm, median relative dose intensities for capecitabine and oxaliplatin were 0.93 and 0.99, respectively. In the FOLFOX-4 arm, the median dose intensities reached 0.99 for both 5-FU and oxaliplatin.
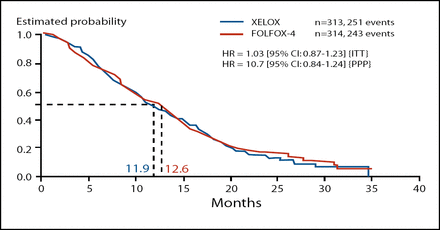
The primary endpoint of this trial was met, though there were slight numerical advantages to the FOLFOX-4 regimen. With regard to PFS, XELOX is non-inferior to FOLFOX-4 as second-line treatment for metastatic colorectal cancer. In the intent-to-treat population, median PFS was 4.7 months in the XELOX group and 4.8 months in the FOLFOX-4 group (HR, 0.97; 95% CI 0.83–1.14). Median OS was also similar in XELOX (11.9 months) and FOLFOX-4 (12.6 months) treatment groups (HR, 1.03; 95% CI 0.87–1.23; Figure 1).
Secondary Endpoint: OS (ITT).
The safety profiles of XELOX and FOLFOX-4 in this trial were similar to those observed in previous studies, with no unexpected toxicities. Thirty-five (35) patients experienced grade 3–4 neutropenia in the FOLFOX-4 arm. By comparison, 5 patients in the XELOX arm experienced grade 3 neutropenia, and no patients experienced grade 4 neutropenia. Hyperbilirubinemia was reported in 34% and 31% of patients in the XELOX and FOLFOX-4 groups, respectively.
A greater proportion of patients in the FOLFOX-4 arm (46%) than in the XELOX arm (38%) withdrew because of insufficient therapeutic response. However, more patients in the XELOX arm (20%) than in the FOLFOX-4 arm (13%) withdrew because of adverse events.
“These findings demonstrate that XELOX is an effective and well-tolerated alternative to FOLFOX-4 as second-line therapy in metastatic colorectal cancer,” Dr. Mace Rothenberg, MD, Vanderbilt Ingram Cancer Center, Nashville, concluded.
The findings of the NO16967 trial support those from a similar study in the first-line setting reported at the ASCO annual meeting [Cassidy J et al. ASCO 2007. Abstract 4030].
Updated Efficacy Results of the MOSAIC Trial, Including Survival, with a Median Follow-Up of Six Years
Approximately one million new cases of colon cancer arise each year worldwide. It has been demonstrated that patients who receive leucovorin (LV)/5-fluorouracil (5FU) after surgical resection of the tumor have better prognoses than those who undergo surgery alone. Recent data suggests that oxaliplatin, in combination with LV5FU, provides an even greater survival advantage. The MOSAIC trial was designed to compare the efficacy of LV5FU to LV5FU plus oxaliplatin (FOLFOX).
The primary endpoint of this study was 3-year disease free survival (DFS); these results were presented in 2003 and were published thereafter [André et al. N Engl J Med 2004]. DFS was chosen as the primary endpoint based on data published by Sargent et al. [Sargent et al. J Clin Oncol 2005] that showed that clinical trials looking at DFS can proceed more rapidly, allowing more timely conclusions of treatment superiorities. Additionally, this study showed that 3-year DFS correlates well with 5-year overall survival (OS). The secondary endpoints were safety and OS.
The 5-year DFS in the MOSAIC study confirms the results observed after 3 years (Table 1) that FOLFOX is superior to LV5FU in this setting. At 5 years, there was a 5.9% difference in favor of FOLFOX treatment over LV5FU. When stratified by disease stage, there was a 7.5% survival benefit with FOLFOX in stage III disease (p=0.005), while there was a non-statistically significant difference of only 3.8% survival benefit with FOLFOX in stage II patients. However, high-risk stage II patients did appear to benefit more from FOLFOX therapy than with LV5FU (Table 2).
DFS in the MOSAIC Trial at 3 and 5 Years.
5-Year DFS Shown By Disease Stage.
The toxicity data, published previously [André et al. N Engl J Med 2004], showed two limiting toxicities with FOLFOX therapy: grade 3 and 4 neutropenia, and grade 3 neuropathy. Long-term safety data showed that there were no differences in the development of secondary cancers between the two arms. Also, there appeared to be a recovery in the degree of peripheral sensory neuropathy; the number of patients with grade three neuropathy decreased by about 15% from the time of treatment out to 4 years.
The probability of surviving at 6 years was 78.6% in the FOLFOX arm and 76% in the LV5FU arm; these results just missed statistical significance at p=0.057. However, these results were again stratified by disease type and patients with stage III disease did fare better on FOLFOX than on LV5FU therapy with statistical significance (p=0.029).
These results, therefore, support the use of FOLFOX as standard adjuvant therapy in colon cancer patients with stage III disease and possibly in patients with high-risk stage II disease. FOLFOX cannot, however, be recommended in low-risk stage II patients. Finally, this 5 and 6 year analysis supports the idea that 3-year DFS data are predictive of overall survival.
The Impact of Dietary Patterns on Cancer Recurrence and Survival in Patients with Stage III Colon Cancer
In this study, Jeffrey A. Meyerhardt, MD, Dana-Farber Cancer Institute, Boston, examined the role of diet in the risk of cancer recurrence and mortality among patients with stage III colon cancer. The dietary analysis was nested within a larger prospective study (CALGB 89803), which compared post-operative IFL and bolus 5FU/LV in patients with stage III colon cancer.
To capture dietary patterns, patients (n=1,009) completed a food frequency questionnaire during adjuvant chemotherapy treatment and 6 months after. Two major dietary patterns were identified. The Western pattern diet was characterized by a higher intake of red meat, fat, refined grains, and desserts. The prudent pattern diet was characterized by higher intake of fruits, vegetables, poultry, and fish.
In the primary analysis of the CALGB 89803 trial, there was no difference in efficacy between the two chemotherapy regimens. Therefore, data from all patients was pooled for the dietary analysis. Patients were evaluated according to quintiles of each dietary pattern and followed for cancer recurrence or death.
The median follow-up was 5.3 years. Patient data was adjusted for gender, age, T and N stage, body mass index (BMI), physical activity level, weight change, baseline performance status, and adjuvant chemotherapy treatment arm.
Among patients who followed the Western pattern diet, those in the higher quartiles were more likely to be male (p<0.0001) and more likely to smoke (p<0.0001). Other baseline characteristics, including age, BMI, level of physical activity, and disease characteristics were similar across quintiles.
Patients who were more likely to follow a Western pattern diet after diagnosis with stage III colon cancer had a poorer long-term outcome, with triple the risk for cancer recurrence or death. Compared to those in the lowest quintile of Western pattern diet intake, those in the highest quintile had a lower disease-free survival (DFS; HR, 3.91; p<0.0001), recurrence-free survival (RFS; HR, 3.14; p<0.0001), and overall survival (OS; HR, 3.75; p<0.0001; Table 1). This pattern was consistent regardless of age, BMI, gender, physical activity level, or treatment arm.
Impact of Western and Prudent Pattern Diets on Colon Cancer Recurrence and Mortality.
Prudent pattern diet did not significantly influence long-term outcome. Patients in the lowest and highest quintiles of prudent pattern diets had similar risk for DFS, RFS, and OS.
Though this trial confirms a link between diet and prognosis, the mechanism driving the association is unclear. For example, smokers and those who adhere to Western diets may develop tumors with a distinct phenotype that worsens prognosis. Behaviors after diagnosis may not necessarily make the difference in outcome.
Deborah Schrag, MD, Memorial Sloan-Kettering Cancer Center, New York, said that the results of this study should change practice. “We need to incorporate diet and exercise recommendations into post-treatment counseling,” she said. “The challenge is to develop educational tools to teach our patients about diet and exercise, and to help motivate them,” she concluded.
- © 2007 MD Conference Express