Summary
Overall survival for patients with head and neck cancers is significantly prolonged with the addition of cetuximab to platinum-based chemotherapy, according to results of the phase 3 EXTREME study. In addition, adding cetuximab did not increase toxicities above those characteristic of platinum-based therapy.
- Oncology Clinical Trials
- Head & Neck Cancers
Overall survival for patients with head and neck cancers is significantly prolonged with the addition of cetuximab to platinum-based chemotherapy, according to results of the phase 3 EXTREME study. In addition, stated investigator Jan Baptist Vermorken, MD, PhD, University Hospital Antwerp, Edegem, Belgium, “Adding cetuximab did not increase toxicities above those characteristic of platinum-based therapy”.
Epidermal growth factor receptor (EGFR) is highly expressed in squamous cell carcinoma of the head and neck (SCCHN), and is an independent prognostic factor for unfavorable local control rates, disease-free survival and overall survival. Cetuximab is an IgG1 monoclonal antibody that specifically targets EGFR and induces antibody-dependent cell-mediated cytotoxicity. Both pre-clinical [Fan Z et al. Cancer Res 1993] and clinical research has shown synergy between cetuximab and platinum-based chemotherapy (cisplatin or carboplatin) with 5-FU (fluorouracil) [Burtness B et al. J Clin Oncol 2005; Bourhis J et al. J Clin Oncol 2006].
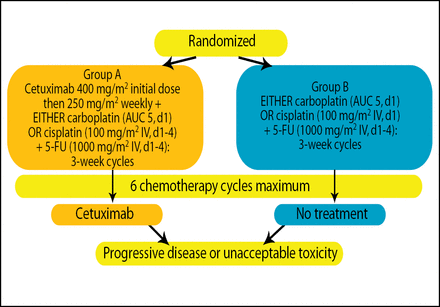
EXTREME was a randomized, phase 3 multicenter study comprised of 442 patients (median age ∼56.5 years; ∼90.5% men) with recurrent or metastatic SCCHN unsuitable for local therapy. Patients were randomly assigned to treatment with cetuximab + platinum (carboplatin or cisplatin) + 5-FU (n=222) or platinum (carboplatin or cisplatin) + 5-FU (n=220) for a maximum of 6 chemotherapy cycles. Patients receiving cetuximab continued on maintenance cetuximab until disease progression or unacceptable toxicity (Figure 1). The primary endpoint was overall survival (OS).
Study Design.
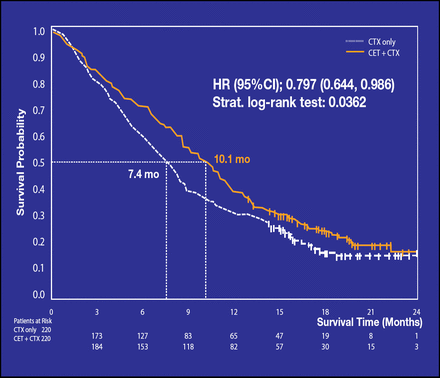
Median OS was significantly longer in the cetuximab plus chemotherapy group (10.1 months vs 7.4 months, respectively; p=0.036; Figure 2).
Overall Survival.
Overall adverse event rates were similar for the two study arms; although acne-like rash and infusion reactions occurred (∼5%) only in the cetuximab group and vomiting (∼5%) and diarrhea (∼5%) were higher with cetuximab-containing therapy. Anemia, neutropenia and thrombocytopenia were higher with platinum-based therapy alone.
Dr. Vermorken concluded, “This is the first systemic treatment in 25 years to show a survival benefit over platinum-based chemotherapy in recurrent/metastatic SCCHN.”
- © 2007 MD Conference Express