Summary
Perioperative chemotherapy provided benefit for patients with colorectal cancer who were treated with surgery for potentially resectable liver metastases. This article discusses the final Results of the EORTC Intergroup Randomized Phase 3 Study 40983 evaluating the benefit of perioperative FOLFOX4 chemotherapy for patients with potentially resectable colorectal cancer liver metastases.
- Gastrointestinal Cancers Clinical Trials
Perioperative chemotherapy provided benefit for patients with colorectal cancer who were treated with surgery for potentially resectable liver metastases. Bernard Nordlinger, MD, Ambroise Paré Hôpital, France, reported this finding on behalf of the European Organisation for Research and Treatment of Cancer.
Dr. Nordlinger noted that liver metastases recur in approximately two-thirds of patients who are treated with surgery alone. He explained that the addition of preoperative chemotherapy would help to reduce the size of liver metastases before surgery. The addition of postoperative chemotherapy would help to kill dormant cancer cells in the remaining portion of the liver. The chemotherapy regimen (6 cycles of FOLFOX4) was chosen because of its response rates of more than 50% among patients with metastatic colorectal cancer.
The study included 364 patients with potentially resectable liver metastases demonstrated on computerized tomography (CT). The patients were randomly assigned to the perioperative chemotherapy and surgery arm (182 patients) or to the surgery alone arm (182 patients). Resection of the liver was actually done in 151 patients in the perioperative chemotherapy arm and in 152 patients in the surgery alone arm. The primary endpoint of the study was progression-free survival (PFS).
Preoperative chemotherapy led to a 29.5% decrease in the size of the liver lesion (from a median of 45 mm to 30 mm). A complete response was achieved in 3.8% of patients and a partial response in 40.1% of patients after preoperative chemotherapy. Disease remained stable in 35.2% of patients. Disease progressed in 6.6%.
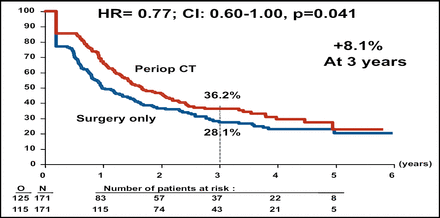
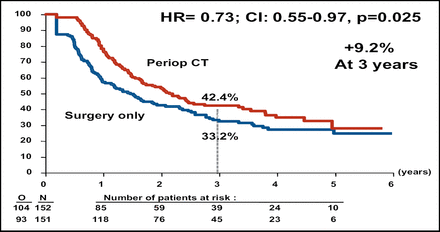
At interim analysis, the Independent Data Monitoring Committee authorized an early release of the final data. Perioperative chemotherapy was associated with better PFS for the total patient population (Table 1). Subgroup analysis demonstrated that better PFS was associated with perioperative chemotherapy for the eligible patient population, or the 171 patients in each arm who had evidence of resectable metastases on CT (Figure 1A). The PFS rate also favored perioperative chemotherapy among the patients who had liver resection (Figure 1B).
Results.
PFS in Eligible Patients.
PFS in Resected Patients.
Preoperative chemotherapy was associated with grade 3 neutropenia (18.1%), diarrhea (8.2%), nausea (3.5%), and sensory neuropathy (2.3%). There was one case of grade 4 febrile neutropenia. Postoperative chemotherapy was also associated with a high incidence of grade 3 sensory neuropathy (9.6%), grade 3 or 4 leukopenia (12.2%), and grade 3 or 4 neutropenia (34.8%). The frequency of postoperative complications was significantly higher for patients who received perioperative chemotherapy (25.2% vs 15.9% for surgery alone; p=0.04). There were higher incidences of biliary fistula, hepatic failure, intra-abdominal infection, and re-operation among patients who received perioperative chemotherapy.
- © 2007 MD Conference Express