Summary
Protein kinase C (PKC) is a family of enzymes involved in phosphorylation and cellular messaging. A growing body of evidence suggests that PKC inhibition may be a possible mechanism to treat bipolar disorder. Tamoxifen, an antiestrogen used in breast cancer treatment, is currently the only PKC inhibitor capable of penetrating the central nervous system.
- Mood Disorders
- Psychopharmacology
Protein kinase C (PKC) is a family of enzymes involved in phosphorylation and cellular messaging. A growing body of evidence suggests that PKC inhibition may be a possible mechanism to treat bipolar disorder. Both lithium and valproate (commonly used mood stabilizers) inhibit PKC (Hahn GG et al. J Psychiatr Res 2005;39(4):355–63). In preclinical models, stimulants have been shown to activate PKC (Einat H et al. BiolPsychiatry 2006;59(12):1160–71) and when compared to normal controls, platelets from patients with bipolar disorder have higher levels of PKC (Friedman E et al. Biol Psychiatry 1993;33(7):520–5). Tamoxifen, an antiestrogen used in breast cancer treatment, is currently the only PKC inhibitor capable of penetrating the central nervous system. In a pilot study of 7 patients with bipolar disorder, tamoxifen significantly decreased manic symptoms (Bebchuk JM et al. Arch Gen Psychiatry. 2000;57(1):95–7). In animal models, tamoxifen normalized stimulant-induced risk taking behaviors and hyperactivity (Einat H. Neuropsychobiology. In press).
Aysegul Yildiz-Yesiloglu, MD, Dokuz Eylul University, Turkey, presented data from a randomized, double-blind, placebo-controlled study of tamoxifen in patients with bipolar disorder conducted at a single center. Subjects aged 18 to 65 with a diagnosis of bipolar disorder and a Young Mania Rating Scale (YMRS) score >20 were eligible to enroll in the study. Subjects were randomized to receive either tamoxifen or matching placebo tablets for 3 weeks. The initial dose of tamoxifen was 20 mg BID, titrated to a target dose of 80 mg/day in divided doses; the placebo group received similar dose adjustments to maintain the blind. Lorazepam was administered if needed to relieve clinical symptoms. Efficacy measures included the YMRS, Hamilton Depression Scale (HAMD), the Montgomery Asberg Depression Rating Scale (MADRS), Clinical Global Impressions, and the Positive and Negative Syndrome Scale (PANSS).
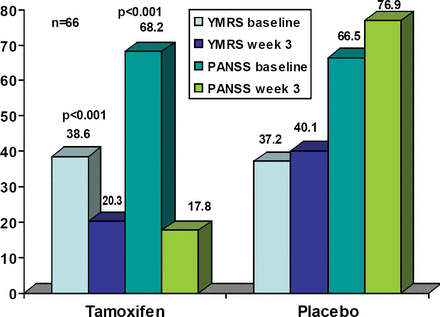
A total of 66 subjects were randomized, 35 to tamoxifen and 31 to placebo. Six (17%) of the tamoxifen group and 10 placebo patients (32%) discontinued from the study, all due to lack of efficacy. There were no statistically significant differences between the treatment groups in any baseline demographic characteristic. There was one suicide attempt in each group. Other reported adverse events in the tamoxifen group were acne (2), flushing on face (1), headache (2), dry skin (1), urticaria (1), and loss of appetite (1). The tamoxifen YMRS and PANSS demonstrated significant improvement at 3 weeks compared to baseline (both p<0.01; Figure 1). The amount of lorazepam used was significantly less in the tamoxifen group at week 3 (p=0.02).
YMRS and PANSS Scores at Baseline and Endpoint.
Although these data are promising, they must be viewed cautiously in light of limitations noted by Dr. Yildiz-Yesiloglu. The placebo response in this study was quite low (approximately 5%) when compared to more typical placebo response rates of 8–1% for single sites. Patients in the tamoxifen group had a significantly higher proportion of medication free days in the month prior to randomization when compared to placebo subjects (p=0.014). The study design required a 24-hour washout of prior medications before randomization, so it is possible that the placebo patients experienced more drug discontinuation effects, especially because more placebo patients were taking psychotropic drugs prior to randomization. Despite these limitations, tamoxifen demonstrated anti-manic effects and PKC pathways remain attractive research targets in bipolar disorder.
- © 2007 MD Conference Express