Summary
One significant issue facing patients currently on antidepressants is the loss of effect that may occur with chronic use of the drug, ie, the so called “poop-out” effect. This article elaborates on this issue and discusses true drug responders versus placebo responders (those that have responded to the non-specific effects of treatment). Despite their response to treatment, there are no available clinical indicators to differentiate between these two types of responders.
- Psychopharmacology
One significant issue facing patients currently on antidepressants is the loss of effect that may occur with chronic use of the drug, ie, the so called “poop-out” effect. Elaborating on this issue, Mark Zimmerman, MD, Brown University School of Medicine, Providence, RI, states, “When you see someone in your practice, and you put them on medication, and they respond, you are essentially seeing two types of individuals. Some are true drug responders. Some have responded to the non-specific effects of treatment. They are placebo responders.” Despite their response to treatment, there are no available clinical indicators to differentiate between these two types of responders.
The fact that some individuals are placebo responders, may account for the majority of cases of relapse reported in medication continuation studies (Zimmerman M, Posternak MA, Ruggero CJ. J Clin Psychopharmacology 2007;27:177–81). One experimental design that is useful in parceling out the rate of relapse attributable to placebo responding is the “Extension Design”. In this paradigm, active medication or placebo is initially assigned in a double-blind fashion. Responders to active medication or placebo then go on to the continuation phase of the study with no change in their treatment. That is, individuals assigned to the medication group remain on medication, while those given placebo continue to receive placebo. This design allows for the question, what percentage of relapse in patients on active medication can be attributable to an initial placebo response?
In order to calculate the percentage of relapse accounted for by placebo responding, four pieces of data are needed. In the acute phase of the experiment, the Response Rate to Medicine (RRM) and the Response Rate to Placebo (RRP) are needed to complete this calculation. In addition, the relapse rate in people who responded to medication (RLM) and the relapse rate in people who responded to placebo (RLP) must be obtained from the continuation phase of the study. Once this data is collected, the rate of relapse that may be attributable to initial placebo responding can be estimated.
In a hypothetical study, 150 patients received medication, and 100 of them responded, (RRM=66%). These 100 medication responders were continued on medication in the continuation phase where 30 patients relapsed (RLM=30%). 150 patients received placebo and 50 of them responded. (RRP=33%) These 50 were continued on placebo in the continuation phase where 25 of them relapsed (RLP=50%)
First, an estimate of the rate at which patients displayed a placebo response despite receiving active medication (ePR) is calculated by dividing the RRP by the RRM.
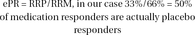
Second, how many patients treated with active drug in the continuation phase, who were responders in the acute phase, are presumptive placebo responders (PPR)? To get this figure, the number of patients receiving active medication in the continuation phase is multiplied by the placebo response rate calculated in the first step.

The number of patients treated with active medication in the continuation phase who have responded, but are expected to relapse because they are presumptive placebo responders (eRLP) can then be calculated by multiplying the PPR by the RLP.
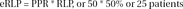
Finally, what percentage of relapse can be attributable to initial placebo responding? This final calculation is obtained by taking the number of presumptive placebo responders on active medication that have relapsed (ie, eRLP) and dividing by the total number of patients who relapsed on medication in the continuation phase.
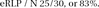
Thus the in this study, presumably 83% of the patients who relapsed after responding to medication and continuing on medication in the continuation phase actually relapsed because they were placebo responders.
Using this method, researchers and clinicians may more accurately determine who is truly responding to their choice of medication versus those who may merely be placebo responders and, thus, more likely to “poop-out” during the latter portion of their treatment.
- © 2007 MD Conference Express










