Summary
This article provides an updated meta-analysis of memantine safety and efficacy for the treatment of moderate to severe Alzheimer's disease, as well as a discussion of the prevalence rates of depression and dementia, comparative safety and tolerability alzheimer's disease treatments, and the need for caregiver psychosocial training.
- Cognitive Disorders
- Dementias
Updated Meta-Analysis of Memantine Safety and Efficacy
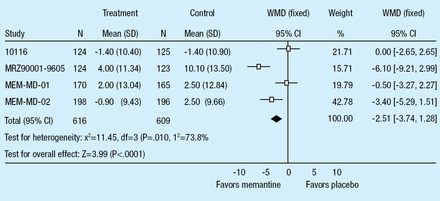
Memantine is an NMDA receptor antagonist approved for the treatment of moderate to severe Alzheimer's disease (AD) in both Europe and the United States. Stephen M. Graham, PhD, and colleagues of the Forest Research Institute presented findings from a recent meta-analysis of randomized, placebo-controlled, double-blind clinical trials with memantine in patients with moderate to severe AD. Trials lasted 16–28 weeks; designs and populations are illustrated in Table 1. With regard to cognition, as measured by the Severe Impairment Battery (SIB), patients on memantine had superior performance than those in the control group, using a last observation carried forward analysis (p<0.0001; Figure 1). The positive treatment effect of memantine was also observed in measures of global functioning (Clinicians' Interview-Based Impression of Change plus Caregiver Input; p<0.001), function (Alzheimer's Disease Cooperative Study-Activities of Daily Living; p=0.003), and behavior (Neuropsychiatric Inventory; p=0.004). Safety analyses indicated a significantly lower rate of all-cause discontinuations in memantine treated patients compared to placebo (p=0.02). The number of memantine-treated patients that discontinued from a study due to an adverse event was also significantly lower than placebo (p=0.04). A comparable number of patients in both treatment groups experienced adverse events (p=0.83) and serious adverse events (p=0.16; Figure 1). Both groups had similar numbers of deaths (2.3% with memantine and 2.5% with placebo; OR=0.94, 95% CI [0.49,1.80]; p=0.84). The authors conclude that these data further support the safety, tolerability, and effectiveness of memantine in patients with moderate to severe AD.
Summary of Methods and Design for Randomized, Double-Blind, Placebo-Controlled Trials of Memantine in Moderate to Severe AD.
Severe Impairment Battery (Last Observation Carried Forward).
One-year Prevalence Rates of Depression and Dementia
Ruby Castilla, MD, DrPH, University of North Carolina, presented results of a study to determine the one-year prevalence of Alzheimer's disease (AD), vascular dementia (VaD), dementia not otherwise specified (NOS), and depressive disorders in patients >60 years of age. The study was conducted using The Integrated Healthcare Information Services Inc. (IHCIS) United States (US) healthcare database which covers 35 health plans, mostly in the Eastern region of the US. Cases ≥60 years with at least one ICD-9 code for dementia (AD, VaD, and NOS) from 01/01/2001 through 12/31/2001 were analyzed. Depressive disorders were defined as follows: atypical depressive disorder, depressive disorder not elsewhere classified, major depressive disorder (recurrent episode), major depressive disorder (single episode), neurotic depression, depressive psychoses, dementia senile with depression, adjustment disorder (depressive), pre-senile depression, artherosclerotic dementia (depressive), and drug depressive syndrome. Patients who died during the course of the year were not included. The control group was comprised of patients >60 years without a diagnosis of dementia who had full records for the year 2001.
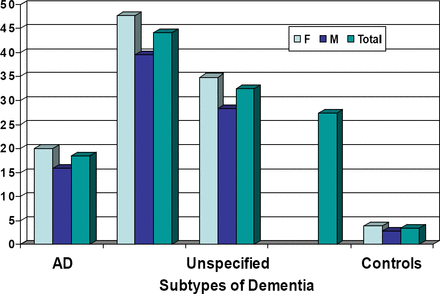
A total of 488,091 patients had a full year of data during 2001. Of this population, 6,440 (1.3%) had a diagnosis of dementia: 2,947 (45.8%) with AD, 725 (11.2%) with VaD, and 2,768 (43.0%) with dementia NOS. Of the patients with dementia, 63.6% were female, with a mean age of 75.7 years, compared with 70.7 years in the control group without dementia. Depressive disorders were more common in the vascular dementia group (44.1%) and the unspecified dementia group (32.4%) than in those patients with AD (18.5%). The control group had a much lower rate of depressive disorders (3.4%).
Study presenters concluded that it is important that patients with dementia, particularly those with vascular dementia, be assessed for depressive symptoms so that appropriate treatment can be initiated.
Prevalence (%) of Patients with Depressive Disorders in all Dementia Cases.
Alzheimer's Disease Treatments: Comparative Safety and Tolerability
Currently approved treatments for Alzheimer's disease (AD) are primarily comprised of cholinesterase inhibitors (ChEIs) such as donepezil, rivastigmine, and galantamine and the NMDA antagonist memantine. Gustavo Aldo, MD, ATP Clinical Research, and colleagues presented results of a comparative analysis of manufacturer data regarding safety and tolerability of these agents. Current prescribing information was obtained from each manufacturer's website in March 2007 and summarized.
The three most frequently reported adverse events for patients that took active drug in double-blind clinical trials are presented in Table 1, and adverse events that led to discontinuation from a pivotal clinical study trial are presented in Table 2. Gastrointestinal symptoms such as nausea, diarrhea and vomiting were the most common side effects for ChEIs. The authors conclude that with regard to ChEIs, gastrointestinal adverse events were the also the most common adverse events that caused patients to withdraw from a trial consistent with the common side effects in that drug class. Memantine, which has a different mechanism of action, had a different adverse event profile compared to the ChEIs, with dizziness, headache, confusion, and constipation being the most common adverse events. Clinicians should keep these side effect profiles in mind when selecting a medication to treat AD.
Three Most Common Adverse Events in Drug-Treated Patients in Clinical Trials
Adverse Events Leading to Clinical Study Discontinuation by Drug
Caregiver Psychosocial Training Decreases Stress
Manuel Martin Carrasco, Clinical Padre Menni, Pamplona, Spain, and colleagues presented recent findings from a study of caregivers of Alzheimer's patients. It is a well-recognized fact that AD caregivers encounter a great deal of stress. The objective of this longitudinal, randomized, parallel group multicenter study was to determine if the administration of the Psychosocial Intervention Programme (PIP) could alleviate some of this stress as measured by the Zarit score, a measure of caregiver burden or stress. Unpaid caregivers >18 years of age with a Zarit score >22 who were living with their patients and taking care of them at least 4 hours/day were eligible for the trial.
The patients they were caring for had to have a diagnosis of dementia of the Alzheimer's type, a Mini-Mental Status Exam score between 10 and 26, be taking rivastigmine for their AD, and have some impairment in activities of daily living. Eligible caregivers were randomized to either: 1) standard care (SC), which consisted of brochures on AD, information by phone when requested, and additional resource information or 2) SC plus PIP, which was comprised of eight (8) 90-minute sessions with a therapist every 1–2 weeks for 4 months, with sessions focused on confronting and solving problems experienced in AD care giving. The primary outcome measure was the change from baseline to final evaluation in the Zarit score. Caregiver psychiatric quality of life was measured by the General Health Questionnaire-28 (GHQ-28) and included as a secondary variable.
A total of 115 caregivers were randomized, 55 to the SC group and 60 to the PIP group, with the majority (90.4%) completing the study. The two groups were comparable demographically. The PIP group had a significantly better response, with a decrease of 8.09 in Zarit score, while the SC group Zarit score increased by 2.08 (p=0.0083), indicating that caregiver burden decreased significantly with administration of the PIP. The GHQ-28 scores also decreased significantly in the PIP group (p=0.0083). The findings of this study indicate that psychosocial training such as PIP may be a beneficial when integrated into the care of AD patients.
- © 2007 MD Conference Express