Summary
Aripiprazole is an atypical antipsychotic approved for the treatment of schizophrenia. Agitation is a common symptom experienced by patients with dementia, and medications with a rapid onset of action and appropriate tolerability in the elderly are desirable in these situations. This article discusses a multicenter, double-blind, placebo-controlled pilot study to determine the tolerability of intramuscular aripiprazole in patients with acute agitation associated with dementia and to determine a maximum tolerated dose.
- Cognitive Disorders
- Dementias Clinical Trials
Aripiprazole is an atypical antipsychotic approved for the treatment of schizophrenia. Agitation is a common symptom experienced by patients with dementia, and medications with a rapid onset of action and appropriate tolerability in the elderly are desirable in these situations. This was a multicenter, double-blind, placebo-controlled pilot study to determine the tolerability of intramuscular (IM) aripiprazole in patients with acute agitation associated with dementia and to determine a maximum tolerated dose.
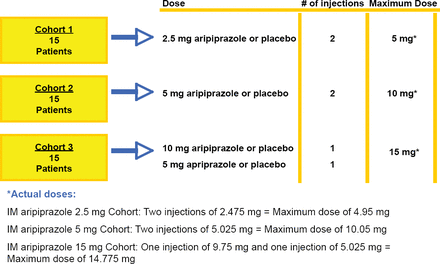
Eligible participants were aged 55–95 years with a diagnosis of Alzheimer's disease, vascular dementia, or mixed dementia. Eligible subjects had to have a PANSS Excited Component (PEC) score between 15 and 32 (inclusive) and a score ≥4 on at least one PEC component (hostility, extreme excitement, poor impulse control, uncooperativeness, tension). The study consisted of a 3 cohort design (Figure 1). Each cohort began with 15 patients, and within each cohort patients were randomized in a 4:1 ratio to receive either active medication or placebo by 2 injections separated by 2 hours. Once an MTD was determined from the 3 cohorts, additional patients were to be enrolled in the MTD cohort until a total of 125 subjects were enrolled. Safety assessments included adverse events, electrocardiograms, vital signs, the Simpson-Angus Scale, Barnes Akathisia Rating Scale, and the Mini-Mental Status Exam (MMSE). Exploratory analyses of efficacy were performed using the PEC, the Agitation-Calmness Evaluation Scale, Clinical Global Impression-Severity, and Clinical Global Impression-Improvement.
Cohort Study Design.
IM aripiprazole 2.5 mg Cohort: Two injections of 2.475 mg = Maximum dose of 4.95 mg IM aripiprazole 5 mg Cohort: Two injections of 5.025 mg = Maximum dose of 10.05 mg IM aripiprazole 15 mg Cohort: One injection of 9.75 mg and one injection of 5.025 mg = Maximum dose of 14.775 mg
Because the tolerability of the 10 mg and 15 mg groups was similar, a maximum tolerated dose was not established. In order to be conservative, additional patients were enrolled in the 10 mg dose group. The only adverse event observed at a higher incidence than placebo in all 3 aripiprazole groups was somnolence, and there were no extrapyramidal symptom-related adverse events (Table 1). One patient from the IM aripiprazole 10 mg group was treated for a probable cerebrovascular event 16 days after taking study drug, but the adverse event was rated as not likely related to the study drug. There was no evidence of changes in vital signs, laboratory measures, or ECGs, and no decline in MMSE score post-dose. Exploratory analyses showed numeric improvement in the efficacy ratings over time although none reached statistical significance. Overall this pilot safety study showed that IM aripiprazole was generally well-tolerated at all 3 doses in acutely agitated subjects with dementia.
Incidence of Treatment-Emergent Adverse Events that Occurred in at Least 5% of Patients in Any Treatment Group.
- © 2007 MD Conference Express