Summary
Agomelatine, a melatonergic agonist and a selective 5-HT2c antagonist, is a novel antidepressant in development for major depressive disorder. This article discusses a meta-analysis of pooled data from three clinical studies. The objective of the meta-analysis was to determine whether gender or baseline severity of depression had any influence on agomelatine efficacy.
- Psychiatry Clinical Trials
- Mood Disorders
Agomelatine, a melatonergic agonist and a selective 5-HT2c antagonist, is a novel antidepressant in development for major depressive disorder (MDD). Alan F. Schatzberg, MD, Stanford University, presented a meta-analysis of pooled data from three clinical studies. The objective of the meta-analysis was to determine whether gender or baseline severity of depression had any influence on agomelatine efficacy.
The three studies included in this exercise were an 8-week dose-finding study comparing 1 mg, 5 mg or 25 mg of agomelatine, placebo, and 20 mg paroxetine and two 6-week flexible dose trials of 25–50 mg agomelatine vs placebo. In the flexible dose trials, agomelatine was increased from 25 mg/day to 50 mg/day if there was no change in Hamilton Depression Rating Scale (HAM-D) at week 2. Eligible subjects were outpatients aged 18–65 with a DSM-IV diagnosis of MDD, a HAM-D total score ≥22, and a Clinical Global Impression severity rating of ≥4 with no comorbid conditions that would interfere with study participation. Other psychotropic medications that could confound study data were prohibited. The primary efficacy measure in all three studies was the change in total HAM-D score from baseline to the final evaluation. For purposes of the meta-analyses, “less severe” was defined as a HAM-D total score ≤ 27 (the median total baseline score), and “more severe” was defined as a HAM-D total score ≥ 27.
A total of 358 subjects received agomelatine and 363 were treated with placebo. There were no statistically significant demographic differences between treatment groups. In the meta-analysis of overall efficacy, agomelatine was significantly better than placebo (p<0.001). Female patients achieved a mean decline in HAM-D score of 13.5 with agomelatine vs an 11.2 decrease with placebo (p<0.001); male patients experienced similar reductions (−13.7 with agomelatine vs −9.9 for placebo; p<0.001). There were no statistically significant differences in efficacy between men and women.
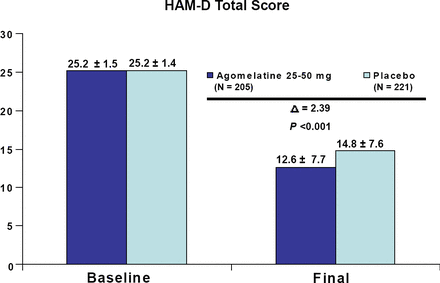
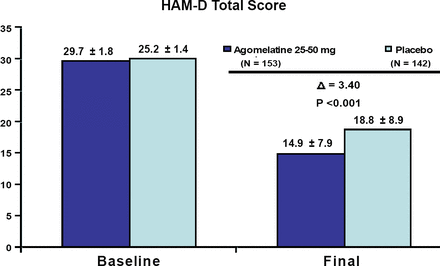
In terms of efficacy and depression severity, agomelatine was significantly better than placebo in both the less severely depressed patients and those with greater symptom severity (both p<0.001; Figures 1 and 2). There was no significant difference in efficacy between the two severity groups; however, additional analyses revealed that the subgroup having the greatest difference from placebo were less depressed males, followed by more severely depressed females. This suggests that men and women may respond differently to the medication depending on their symptom severity. Agomelatine efficacy results were recently published online on May 4, 2007 by Pierre and Kasper, in the International Journal of Neuropsychopharmacology.
HAM-D Total Score in Patients with Less Severe Depression (HAM-D<=27). Meta-Analysis of 3 Placebo-Controlled Studies.
HAM-D Total Score in Patients with More Severe Depression (HAM-D>=27). Meta-Analysis of 3 Placebo-Controlled Studies.
- © 2007 MD Conference Express