Summary
This article presents data from the Investigation of Transdermal Exelon in Alzheimer's disease [IDEAL] trial. Rivastigmine is an acetylcholinesterase inhibitor approved for the treatment of mild to moderate dementia in both Alzheimer's disease and Parkinson's disease. Rivastigmine is currently available in capsule formulation, and its pharmacological characteristics are compatible with transdermal delivery. The IDEAL study was conducted to determine whether the efficacy and safety of a transdermal formulation of rivastigmine would be equivalent to the oral formulation, and to collect long-term safety data.
- Extrapyramidal & Movement Disorders
- Cognitive Disorders
- Dementias Clinical Trials
George Grossberg, MD, St. Louis University, presented data from the Investigation of Transdermal Exelon in Alzheimer's disease (IDEAL) trial. Rivastigmine (Exelon) is an acetylcholinesterase inhibitor approved for the treatment of mild to moderate dementia in both Alzheimer's disease (AD) and Parkinson's disease. Rivastigmine is currently available in capsule formulation, and its pharmacological characteristics are compatible with transdermal delivery. Advantages associated with transdermal therapy include better compliance, greater consistency in plasma levels of drug, and a decrease in time to maximal therapeutic concentrations (Cevc G Expert Opin Investig Drugs 1997;6:1887–1937). The IDEAL study was conducted to determine whether the efficacy and safety of a transdermal formulation of rivastigmine would be equivalent to the oral formulation, and to collect long-term safety data.
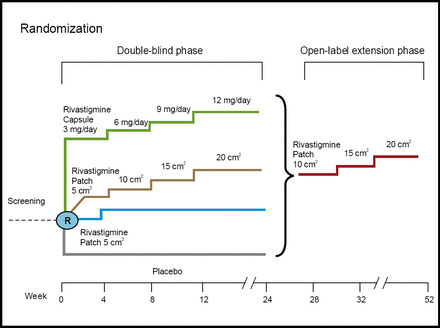
Patients aged 50–85 with a diagnosis of AD were included in the trial, which consisted of a 24-week randomized, double-blind, placebo-controlled phase, followed by a 28-week open-label extension phase (Figure 1). The primary efficacy measures were the Alzheimer's disease Assessment Scale-cognitive subscale (ADAS-cog) and the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change (ADCS-CGIC). Skin irritation and patch adhesions were systematically assessed.
IDEAL Study Design.
A total of 1,195 patients were randomized to treatment in the double-blind phase, with 970 (81%) completing all 24 weeks; 870 (73%) patients continued into the open-label phase. During the double-blind phase of the study, 83.8% of patients in the 10 cm2 patch group met the target dose of study drug for ≥8 weeks, compared to 49.5% of the capsule group and 53.1% of the 20mg2 groups. At the end of the 24-week double-blind phase, the rivastigmine 10 cm2 patch, 20 cm2 patch, and capsule treatment groups were significantly better than placebo in ADAS-cog, ADCS-Activities of Daily Living scale, Mini-Mental Status Exam, Trail Making Test A, and ADCS-CGIC (all p<0.05), with the exception of the 20 cm2 patch in the ADCS-CGIC (p=0.054). During the open-label phase, 72.6% of participants achieved the target 20 cm2 patch. At the end of the open-label phase, patients who received rivastigmine in any form during the double-blind treatment period had small declines in the ADAS-cog when compared to baseline (−0.3); those taking placebo in the double-blind phase had a −0.9 change in ADA-cog scores compared to baseline. The most common adverse events associated with rivastigmine were nausea, vomiting, and diarrhea; The 10 cm2 rivastigmine patch had 3 times fewer nausea and vomiting adverse events compared to rivastigmine capsule (Table 1). The open-label phase adverse events were similar to those reported in the double-blind phase. Patients taking placebo in the double-blind phase had a higher incidence of adverse events when switched directly to the 10 mg2 patch, suggesting that naïve patients should be titrated using a 5 cm2 patch. Local skin irritation led to study discontinuation in 2.4% of the 10 cm2 group and 3.7% in the 20 cm2 group. The majority of patients (>90%) experienced “none, slight, or mild” skin irritation as their most severe skin reaction during the study. Ninety-six percent (96%) of caregivers in the 10 mg2 group reported good adhesion over 24 hours, with the patch staying completely on or just starting to lift up at the corners. The conclusion of the study was that transdermal rivastigmine treatment over one year was a convenient, effective, and well-tolerated medication delivery method in patients with AD.
Most Frequently Reported Adverse Events (n, %) During the Double-Blind Phase and During the First Four Weeks of the Open-Label Extension Phase Presented by the Patient's Double-Blind Phase Treatment Group.
- © 2007 MD Conference Express