Summary
Borderline personality disorder (BPD) affects approximately 2% of adults, and 11% of patients seeking psychiatric treatment. Previous reports suggested that olanzapine (OLZ) may be useful in the treatment of this disorder. This article presents the results of the double-blind phase of a clinical trial of OLZ in patients with BPD.
- Personality Disorders Clinical Trials
Borderline personality disorder (BPD) affects approximately 2% of adults, and 11% of patients seeking psychiatric treatment. Previous reports suggested that olanzapine (OLZ) may be useful in the treatment of this disorder. Charles Schulz, MD, University of Minnesota, presented the results of the double-blind phase of a clinical trial of OLZ in patients with borderline personality disorder. This was a 12-week, randomized, double-blind, placebo-controlled trial of a flexible dose of OLZ (2.5 to 20 mg/day). Patients were diagnosed with BPD; patients with comorbid psychiatric conditions such as bipolar disorder, or substance dependence were excluded. Investigators randomized 314 patients at sites in the United States and Western Europe. In the 12-week double-blind phase, subjects were treated with either OLZ 2.5 to 20 mg/day or placebo. Subjects had the opportunity to enter an open-label phase where they received OLZ 2.5 to 20 mg/day for an additional 12 weeks. The primary outcome measure was a new rating scale, the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD). Specifically developed for BPD, this scale is a semi-structured interview and encompasses 9 items which are scored from 0 to 4: inappropriate anger, affective instability, emptiness, identity disturbance, paranoia/disassociation, fear of abandonment, self-injury, impulsivity, and unstable interpersonal relationships.
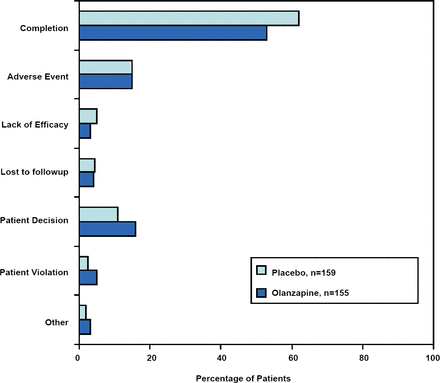
A total of 155 patients received OLZ and 159 received placebo. The majority of the subjects were women (72.9% and 69.2% of the OLZ and placebo groups, respectively). Thirty-three percent (33%) of the OLZ subjects and 44% of the placebo subjects were unemployed due to their disease. “This group of patients, to my estimation, was probably relatively substantially ill… over a quarter checked off the item on their demographic sheet that the illness they had (BPD) did not allow them to be competitive in employment,” commented Dr. Schulz. The completion rate was over 50% in both groups (Figure 1). “When we launched these studies, many people we consulted with…felt that BPD patients could not complete a trial. When you think about the completion rates in schizophrenia, bipolar disorder and other serious psychiatric illnesses which may be around 50% or 45%, this is actually a relatively nice completion rate, especially for a 12-week study” said Dr. Schulz.
Patient Disposition with Reasons for Discontinuation.
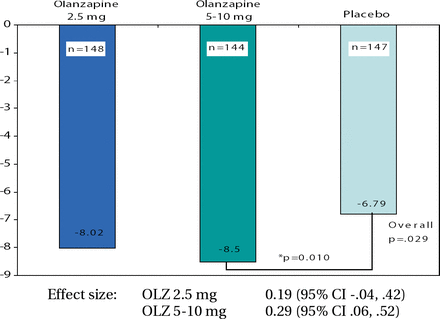
In terms of efficacy, the study did not meet its primary endpoint as both groups demonstrated clinically meaningful improvement in ZAN-BPD scores. The mean change from baseline to final was −6.56 with OLZ compared with −6.25 with placebo (p=0.661). It is entirely possible that the study interventions (which included regular contact with study staff and review of symptoms) led a high placebo response. When a responder analysis was performed, the OLZ group (64.7%) demonstrated a trend compared to placebo (53.5%; p=0.062). Responders were defined as any patient with a 50% reduction in ZAN-BPD score from baseline to any other visit. “Those of you who may have worked in clinical trials with BPD patients may have experienced where subjects say they really appreciate the psychoeducational parts of the informed consent, the review of their symptoms, and how they are doing over time. Even though it's a placebo as far as the medicine is concerned, it's not without positive interpersonal effect,” concluded Dr. Schulz. Mary Zanarini, MD, Harvard University, presented the results from a second trial of OLZ in BPD. The eligibility criteria were the same as the aforementioned study except that this was a dose comparison study. The treatment groups were OLZ 2.5 mg (n=150), OLZ 5–10 mg (n=148) and placebo (n=153). A total of 451 patients were randomized at sites in the United States, South America, and Eastern Europe. The demographic characteristics were comparable among the 3 treatment groups, with approximately 20% being unemployed due to their BPD. This study also had an impressive retention rate, with over 60% in each treatment group completing the trial. The OLZ 5–10 mg group was superior to placebo in change from baseline to final in ZAN-BPD score (p=0.010; Figure 2). The safety profiles in both studies were consistent with previous trials of OLZ in adults, and results from these studies led the presenters to conclude that a 5–10mg/day dose of OLZ may be effective in the treatment of BPD. “The high proportion of subjects completing this study demonstrate the feasibility, especially considering the length of the study,” reiterated Dr. Zanarini. However, due to the high placebo response to study procedures, future trial designs in this population should consider the placebo arm as an active comparator.
ZAN-BPD Total Change from Baseline to Endpoint (LOCF) – Primary Efficacy Analysis.
- © 2007 MD Conference Express