Summary
In 2006, news of increased late thromboses with drug-eluting stents compared with bare metal stents caused great concern among the medical community worldwide. Since then, experts in the field, including the United States Food and Drug Association, have scrutinized the data and have made recommendations for delivering the safest and most efficacious percutaneous coronary intervention.
- Interventional Techniques & Devices
- Thrombotic Disorders
In 2006, news of increased late thromboses with drug-eluting stents (DES) compared with bare metal stents (BMS) caused great concern among the medical community worldwide. Since then, experts in the field, including the US Food and Drug Association (FDA), have scrutinized the data and have made recommendations for delivering the safest and most efficacious PCI. These concepts were summarized in a special session at the American College of Cardiology (ACC).
Pooled data from the industry-sponsored randomized trials (TAXUS I, II, IV, V, VI; RAVEL; SIRIUS; E-SIRIUS; C-SIRIUS) indicate that very late stent thrombosis (>12 months) is real, though rare, said John McB. Hodgson, MD, St. Joseph's Hospital and Medical Center, Phoenix, Arizona. At 36 months, freedom from late thromboses was 98.7% with TAXUS versus 99.2% with bare metal stents (BMS), for a difference of 0.46%. With CYPHER, the rates were 98.9% versus 99.4% with BMS, for a difference of 0.57%, he said.
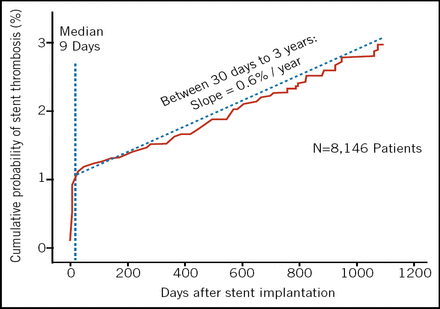
These data, however, only discuss risk in a controlled setting. Risk in the “real world” may be a bit higher, according to the recent Bern-Rotterdam cohort study of 8,146 patients (Lancet. 2007;369:667–78) in which 152 angiographic DES thromboses occurred between 30 days and 3 years, for a rate of 2.9% and a slope of 0.6% per year (Figure 1).
Real World Data. DES Stent Thrombosis Bern-Rotterdam Cohort Study.
“This confirmed that an increase in late stent thrombosis is occurring well after the range we were used to with bare metal stents,” Dr. Hodgson observed. “However, we should keep in perspective that the frequency of events is actually very low.”
The fact that the curve keeps sloping upward particularly concerns Martin Leone, MD, of Columbia University, New York, who noted that the available data have not revealed a point at which late thromboses level off.
Nevertheless, while “the signal is unmistakable,” he agreed that the actual numbers are small. A recent study by Stone et al (New Engl J Med. 2007;356:998–1008) reported a total of 20 early and late events with TAXUS versus 14 with BMS out of 3,513 patients; and 10 events with CYPHER versus 5 with BMS out of 1,748 patients, over a 4-year period. There is evidence that rates are higher in off-label uses, probably around 2.5%.
Whether an increase in mortality parallels the excess in late thromboses remains unclear, since some but not all studies have linked these endpoints, speakers said. In diabetics, however, the mortality risk with DES seems higher, according to a recent pooled analysis (New Engl J Med. 2007;356:989–97), which found 4-year survival to be 95.6% with BMS versus 87.8% with DES in that population. The Academic Research Consortium found that stent thromboses usually manifest as death and myocardial infarction (New Engl J Med. 2007;356:1020–9). However, the overall mortality risk associated with DES is still being clarified.
The 2006 findings initially resulted in a slight reduction in use of DES in the United States; penetration is at 70% now, down from a peak of 89%. Use of DES in Western Europe has remained fairly steady at 53% (from 26% in Sweden to 95% in Switzerland). Japan peaked at 72% in 2005, where it has remained, in spite of the controversy, according to Gregory J. Dehmer, MD, Texas A&M College of Medicine, College Station, Texas.
Understanding Where the Risk Lies
After much consideration of the data, an FDA Review Panel concluded:
DES are safe and effective when used for on-label indications
Use for off-label indications remains a concern; and should be discouraged if long-term antiplatelet therapy is not possible
More long-term real world data are needed as the association with long-term increased rates of death or MI is uncertain
Dual antiplatelet therapy should be continued for 12 months in patients not at high risk for bleeding
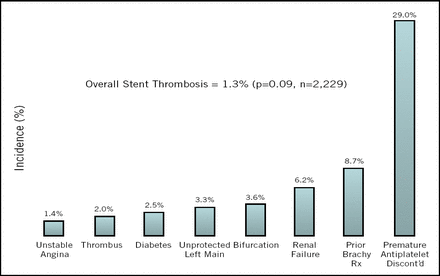
Knowing the predictors of stent thrombosis can help guide treatment decisions, speakers said. The main problems are premature early discontinuation of antiplatelet therapy (Figure 2) and an increase in events following the completion of short (<1 year) courses of thienopyridiene therapy. In the Bern-Rotterdam study of 8,141 patients, 61 developed late stent thrombosis. Of those, 26% were on no antiplatelet therapy, 51% were on a single agent, and 23% were on dual therapy, said Bernhard Meier, MD, of the Swiss Cardiovascular Center, Bern.
Early Discontinuation of Antiplatelet Therapy is the Strongest Risk Factor of ST.
In Dr. Leone's opinion, “Off label use is the critical issue, as this represents 65% to 85% of our cases of stent thrombosis!”
Other predictors of DES thrombosis are advanced age, acute coronary syndrome, diabetes, low ejection fraction, prior brachytherapy, and renal failure. Angiographic features include multiple lesions, small vessels, ostial or bifurcation lesions, the use of long stents or overlapping stents, and suboptimal stent results.
All of the speakers emphasized the need for patients to comply with 12 months of dual antiplatelet therapy and stressed that these discussions should occur before the procedure. In patients not expected to be compliant, DES use should probably be avoided, said Dr. Hodgson. He advised clinicians to “stent the right patient and use DES for the right indications.” When done for proper indications, he said, “the risk/benefit ratio still favors DES.”
Dr. Leone said he determines the relative value of DES versus BMS in every patient and has abandoned unrestricted use of DES. He balances the value of dual antiplatelet therapy against the risk of bleeding (1% per year), and usually avoids DES in patients with a history of bleeding, upcoming surgery, certain concomitant medications, and socioeconomic factors affecting compliance. His advice was, in patients with increased restenosis risk, consider DES; in patients with safety concerns, consider not using DES. Practice good implantation techniques and consider intravascular ultrasound (IVUS) guidance.
Is More CABG the Answer?
More consideration of coronary artery bypass grafting (CABG) in selected patients was proposed by Craig R. Smith, MD, of Columbia University, New York. With elective CABG, survival is improved, revascularizations are fewer, and symptom relief is better compared with stenting, when multiple vessels are involved, he said.
In general, “the more critical the anatomy, the more CABG excels,” Dr. Smith maintained. CABG is often preferable in patients with 3-vessel disease with proximal left anterior descending artery (LAD), multiple sites, and left main disease. Optimal use of arterial conduits is critical for success, he said.
Looking to the Future
Ron Waksman, MD, of Georgetown University, Washington, DC, previewed the next generation of DES. These devices will address challenging anatomic subsets, with a focus on pro-healing and fast re-endothelialization that will result in decreased smooth muscle activation and reduced collagen secretion—which will optimize the healing response. Novel carriers will include thinner biostable polymers, bioabsorbable polymers, or no polymers, and surface modifications. Drugs will be gentler and will include anti-inflammatory and immunosuppressive agents. In early studies of novel stents, late stenosis risk has been essentially non-existent. New DES programs are underway by more than a dozen manufacturers.
- © 2007 MD Conference Express