Summary
Increased levels of high-density lipoprotein cholesterol decrease the risk of cardiovascular disease. Torcetrapib, a cholesteryl-ester-transfer protein inhibitor, has a potent effect on increasing HDL-C levels that was hoped would translate into the halting or reversal of atherosclerosis. The results from three clinical trials of torcetrapib are discussed in this article.
- Cardiology Clinical Trials
- Lipid Disorders
Increased levels of high-density lipoprotein cholesterol (HDL-C) decrease the risk of cardiovascular disease. Torcetrapib, a cholesteryl-ester-transfer protein (CETP) inhibitor, has a potent effect on increasing HDL-C levels that was hoped would translate into the halting or reversal of atherosclerosis. The results from three clinical trials of torcetrapib were presented by Steven Nissen, MD, FACC, president of the American College of Cardiology, and John Kastelein, MD, PhD, of the Academic Medical Center in Amsterdam, The Netherlands.
Dr. Nissen reviewed the results of the ILLUSTRATE trial, which was terminated prematurely in early December 2006 due to an excess in total mortality in patients who were randomized to torcetrapib. A total of 1,188 patients participated in the ILLUSTRATE study at 137 centers in the United States and Europe. Intravascular ultrasound (IVUS) was performed on study subjects, who were then treated with atorvastatin to decrease levels of low-density lipoprotein-cholesterol (LDL-C) to <100 mg/dL. Subjects were subsequently randomized to treatment with atorvastatin monotherapy or atorvastatin combined with torcetrapib 60 mg/day. After 24 months of treatment, IVUS was repeated in 910 subjects (77%). The primary efficacy measure was change in the percent atheroma volume.
There were no significant differences in any of the baseline demographic variables. The torcetrapib-atorvastatin group (n=464) had a significant increase in HDL-C, significant decrease in LDL-C, and a decrease in LDL-C/HDL-C ratio (all p<0.001) compared with the atorvastatin monotherapy group (n=446; Table 1). According to Dr. Nissen, this study exhibited the lowest magnitude of LDL-C/HDL-C ratio ever observed. The change in percent atheroma volume increased by 0.19% in the atorvastatin-only group and by 0.12% in the torcetrapib–atorvastatin group (p=0.72). The HDL levels increased slowly, over 6–9 months after randomization.
Final Lipid Values and Percentage Change.
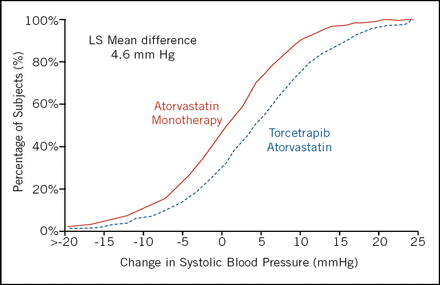
Unfortunately torcetrapib did not slow the progression of atherosclerosis, nor were there any significant differences in secondary measures. Torcetrapib-treated patients experienced a mean increase of 4.6 mm in systolic blood pressure (Figure 1). The main trial was stopped because of an increase in all-cause mortality of approximately 60% in the torcetrapib/atorvastatin group when compared with the atorvastatin group. “We just don't know what the toxicity is, and that makes it difficult to interpret the trial,” summarized Dr. Nissen.
Cumulative Histogram Change in Systolic Blood Pressure.
Dr. Kastelein presented the results from the RADIANCE 1 and RADIANCE 2 studies. Both studies sought to determine the change in atherosclerosis, using imaging, after treatment with either torcetrapib plus atorvastatin or with atorvastatin alone. RADIANCE 1 was conducted in patients with heterozygous familial hypercholesterolemia (HeFH) and RADIANCE 2 was conducted in patients with mixed hyperlipidemia. These patient populations were selected because they tend to have low levels of HDL-C and high levels of LDL-C. Subjects were treated with atorvastatin to reduce their LDL-C to goal, and then randomly assigned to one of the two treatment arms. The studies were conducted in 8 countries, and scans were centrally read in Europe and the United States. The primary outcome measure of both studies was change in the maximum carotid intima-media thickness (max CIMT).
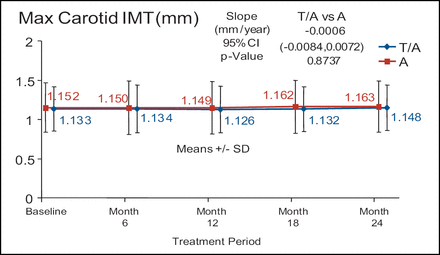
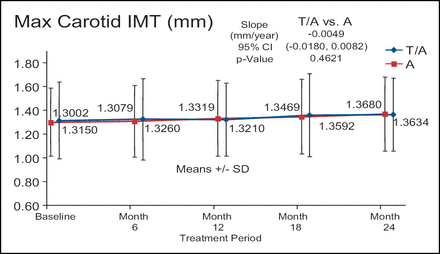
There was no significant difference in atherosclerotic progression in the torcetrapib/atorvastatin treatment arm (n=450) compared with the atorvastatin monotherapy arm (n=454) despite a 52% increase in HDL-C and a 21% decrease in LDL-C (Figure 2). In addition, the torcetrapib/atorvastatin arm had approximately twice as many serious cardiovascular events when compared with the atorvastatin monotherapy arm (5.3% vs 2.4%, respectively). In the RADIANCE 2 trial, 377 patients were treated with torcetrapib/atorvastatin and 375 patients were treated with atorvastatin alone. There were no differences in any of the arms at any time point. Dr. Kastelein described the graph of the max CIMT of the two treatment arms over time as flat. (Figure 3).
RADIANCE 1 – Heterozygous FH.
RADIANCE 2 – Mixed Dyslipidemia.
Both investigators emphasized that although development has ceased on this particular compound, the class of drugs still holds promise.
- © 2007 MD Conference Express