Summary
Thirty million people worldwide take non-steroidal anti-inflammatory drugs (NSAIDS) for the treatment of chronic pain and inflammation. In light of the cardiovascular (and renal) risks associated with NSAIDS, this article discusses whether clinicians safely use these drugs in their practices.
- Inflammatory Disease
Thirty million people worldwide take non-steroidal anti-inflammatory drugs (NSAIDS) for the treatment of chronic pain and inflammation. In light of the cardiovascular (and renal) risks associated with NSAIDS, can clinicians safely use these drugs in their practices?
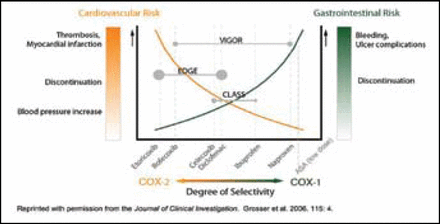
The spectrum of biological effects with NSAIDS depends on the selectivity of cyclooxygenase (COX) inhibition. COX-1 inhibitors pose gastrointestinal (GI) toxicity but may have antithrombotic effects. COX-2 inhibitors may have less GI toxicity but can have prothrombotic potential, which seems to differ across individual drugs within the coxib class (Figure 1). Cardiovascular risk may be dose-related and possibly duration-related, said Debabrata Mukherjee, MD, of the Gill Heart Institute, University of Kentucky, Lexington.
Implications of Relative Degrees of Selectivity.
James Brophy, MD, of Westmount, Canada, who explored the post-marketing data on NSAIDS, described an important meta-analysis published last year (Br Med J. 2006;332:1302–8) in which the relative risk for cardiovascular events for all COX-2 inhibitors was increased by 42% compared with placebo. Individual differences were difficult to show. “There are 121 randomized controlled trials,” he remarked, “but we still have outstanding questions.”
Observational studies help to fill this gap. The largest studies all show increased cardiovascular risks with rofecoxib (Vioxx®) (14% to 80%), but results are inconsistent for celecoxib (Celebrex®), particularly in standard doses. Patients without previous myocardial infarction (MI) have a 23% increased risk with rofecoxib but no increased risk with celecoxib; in patients with previous MI, however, risk is increased by 59% with rofecoxib and by 40% with celecoxib.
Dr. Mukherjee agreed. “The totality of the data suggests that celecoxib is not worse than the older NSAIDS though there is a signal of risk at higher doses,” he said. “The black box warning is for all the coxibs. If you need an NSAID, naproxen may be the least toxic.”
Unresolved questions might be answered by the Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen (PRECISION) trial, which will assess the relative cardiovascular safety of three of the most commonly used pain relievers. The study will enroll patients with arthritis and either coronary heart disease or multiple risk factors for heart disease, and follow them for the occurrence of cardiovascular events.
Managing the Cardiac Patients Who Needs NSAIDS
Elliott Antman, MD, of the Brigham and Women's Hospital, Boston, said that in his practice he uses NSAIDS only as necessary and in patients at the lowest cardiovascular risk, in the lowest possible doses, using the lowest risk agents and the shortest duration of treatment. In patients deemed to have no risk for substance abuse, short-term narcotics may actually be a better choice, he added.
In a study reported at the American Heart Association 2006, Gibson, et al showed that among patients suffering an ST-segment-elevation MI, the adjusted risk for death or MI was increased by 29% in patients who were taking NSAIDS within the prior week. Dr. Antman advised clinicians to be sure their MI patients were not continued on NSAIDS when admitted.
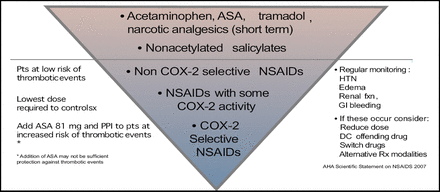
He commented, “COX-2-selective NSAIDS should not be the first line but the last line of treatment today,” advising clinicians to closely monitor patients should they prescribe NSAIDS (Figure 2).
Pharmacologic Therapy for Musculoskeletal Symptoms in Patients with Known CVD or Risk Factors for IHD.
NSAIDS and the Kidney: What is the Danger?
Michael E. Farkouh, MD, Mt. Sinai Heart Clinical Trials Unit, New York, emphasized that dose-dependent renal effects also occur in up to 5% of patients following long-term use of NSAIDS. In light of the coxib controversy, concerns over renal toxicity are increasing, he said.
Problems include acute renal failure, hypertension, congestive heart failure, fluid and electrolyte abnormalities, nephritic syndrome, and papillary necrosis. Patients most at risk include those with age-related decline in glomerular filtration rate, hypovolemia, loop diuretic use, heart failure, cirrhosis and nephrosis.
Risk for acute renal failure with NSAIDS varies by the preparation, according to a recent epidemiologic study (Am J Epidemiol. 2006;164:881–9). Naproxen and rofecoxib carry the highest adjusted relative risk over non-exposure (about 2.3 fold); celecoxib's risk is 1.5 and meloxicam's risk is nearly 1.3. Multiple studies have demonstrated that adverse renal effects with rofecoxib are dose-related.
Another significant problem is the aggravation of hypertension with NSAIDS. When patients require treatment with both antihypertensive agents and NSAIDS, Dr. Farkouh advised, “Don't disregard the blood pressure effects of these drugs and make sure your office measures blood pressure reliably.” He recommended using lower doses of the NSAID (nonselective or coxib), titrating the antihypertensive, reducing salt intake, questioning patients about over-the-counter NSAID use, and considering aspirin or a non-opioid analgesic instead.
In contrast to current agents, a new agent, lumiracoxib (Prexige®), has a greatly improved renal safety profile and, especially at low doses, is associated with less heart failure compared with other agents, Dr. Farkouh said.
- © 2007 MD Conference Express