Managing Opportunistic Infections
Resistant and non-responsive opportunistic infections continue to be a clinical challenge for the practising physician. During the recent 46th Interscience Congress on Antimicrobial Agents and Chemotherapy, John Bennett, MD from the National Institutes of Health chaired an informative satellite symposium on evolving treatment strategies. This CME program presented current evidence on the management of invasive pulmonary mold infections (K A Marr, MD, University of Washington School of Medicine), invasive candidiasis (J Bennett, MD, National Institute of Allergy and Infectious Disease), community-acquired pneumonia (CAP) (T File Jr, MD, Northeastern Universities College of Medicine) and treatment options for deep-tissue MRSA infections (R Moellering Jr, MD, Harvard Medical School).
Invasive Mould and Fungal Infections
Mould and fungi are increasingly recognised as major pathogens in critically ill patients. Candida spp. and Cryptococcus spp. are the yeasts most frequently isolated in clinical practice. The most frequent filamentous fungi (moulds) isolated are Aspergillus spp., but Fusarium spp., Scedosporium spp., Penicillium spp. and zygomycetes are increasingly seen. The increase in invasive fungal infections can be attributed to the use of antineoplastic and immunosuppressive agents, broad-spectrum antibiotics, prosthetic devices and grafts, and more aggressive surgery. Patients with burns, neutropenia, HIV infection and pancreatitis are also predisposed to fungal infection.
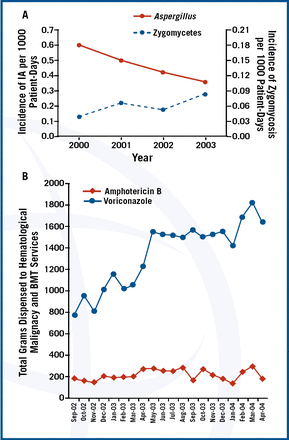
Invasive mould infections (IMIs) should be suspected in neutropenic or immunosuppressed patients or those on corticosteroids. In these patients, the data indicates that therapy with variconazole should be started immediately because of an improvement in survival rate at 12 weeks compared to amphotericin B (70.8% vs. 57.9% [hazard ratio, 0.59; 95% CI 0.40 to 0.88])1. However, there is increasing recognition of zygomycosis in neutropenic patients2
Kontoyiannis, D.P., M.S. Lionakis, R.E. Lewis, et al. Zygomycosis in a tertiary care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis, 2005. 191(8):p. 1350–60.
The shift in etiology could be due to selection of resistant organisms by variconazole. Dr. Marr asked “Should amphotericin formulation or posaconazole be started instead?”
Initial data from a study of posaconazole as salvage therapy, suggest that posaconazole may be an alternative for treating zygomycosis3. She reported the results from a 2004 salvage therapy study4, in which patients received either voriconazole or a combination of voriconazole and caspofungin. Mortality from aspergillosis was lowest in patients receiving the combination regimen (hazard ratio, 0.42; 95% CI, 0.17–1.1; p=.048).
At the end of her presentation Dr. Marr showed new data on Rhizopus spp.5 (Figure 3), which indicates that dead fungus can damage cells. This may explain the lack of efficacy of fungicidal agents in clinical disease.
Rhizopus mold.
The role of newer agents in the management of invasive candidiasis was reviewed by Dr. Bennett. Until now, treatment options for invasive mycoses have been primarily amphotericin B and the azoles (fluconazole and itraconazole). These traditional agents are limited by an inadequate spectrum of activity, drug resistance, toxicities, and drug-drug interactions.
The approval of new echinocandins (caspofungin, micafungin, anidulafungin) and an azole (voriconazole), has expanded the armamentarium. According to the findings of a 2002 New England Journal of Medicine study, caspofungin is equivalent in efficacy, but less toxic than, amphotericin B in the treatment of candidemia, with successful outcomes in 73.4% of patients vs. 61.7%, respectively (difference after adjustment for APACHE II score and neutropenic status, 12.7 percentage points; 95.6 percent confidence interval, −0.7 to 26.0)6. Similarly, Micafungin has been found to be equivalent to Liposomal Amphotericin B (L-AMB) with advantages in terms of renal toxicity and adverse events7. Anidulfungin is not metabolised in the liver or kidney, reducing the likelihood of drug interactions8 and is equivalent to fluconazole in the treatment of esophageal candidiasis9.
Differences between the echinocandins are small, making the choice of agent difficult. In non-neutropenic hemodynamically stable patients not colonized with Candida glabrata or C krusei, fluconazole remains the treatment of choice. In patients with poor renal function, the echinocandins are an alternative. In neutropenic patients, amphotericin B remains the gold standard.
For salvage therapy, Dr. Bennett stated that there was a paucity of data in neutropenics regarding the use of echinocandins or azoles. He concluded that treatment choices for invasive candidiases were complex. Voriconazole has become the primary treatment for most forms of invasive aspergillosis in both North America and Europe, and is included in a number of guidelines10⇓⇓-13. Posaconazole offers a broad antifungal spectrum. Echinocandins are fungicidal against most Candida species14. However, significant questions remain, including the management of breakthrough infections, treatment failures, and the efficacy of the new antifungal agents against less common fungi.
CAP in Hospitalized Patients
Guidelines for the management of community-acquired pneumonia (CAP) were initially published in the early 1990′s. The most recently published guidelines for CAP are the 2003 Infectious Diseases Society of America (IDSA) update15 and the European Respiratory Society (ERS) 2005 guidelines16. In North America, the CAP Guidelines are currently under revision. Dr. File reviewed the topics being reviewed for inclusion in the new North American (ATS/IDSA) guidelines which include: the admission decision, diagnostic testing, risk factors and specific pathogens, empiric antibiotic therapy, adjunctive therapy, and performance measures.
All of the guidelines recommend that physicians use prediction tools to support, not replace, clinical judgment. Severity and need for inpatient care can also be influenced by other factors, such as whether the patient is immunocompromised, social factors, multilobar involvement, or the presence of advanced lung disease. These considerations also influence the decision to admit to hospital or the intensive care unit (ICU).
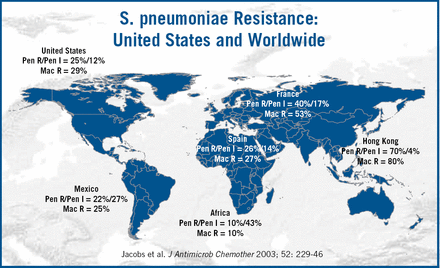
Currently, the American guidelines recommend chest X-ray (CXR), use of epidemiologic data, and Gram stain prior to therapy. The American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) Guidelines17 recommend urinary antigen testing and blood culture with severe illness, whereas the ERS guidelines16 recommend blood cultures for all hospital admissions. Health-care associated pneumonia has recently been included in the ATS Guidelines for hospital and ventilator associated pneumonia15, recognizing that institutionalised patients have many similar risk factors to those with hospital-acquired pneumonia. Differences in the guidelines reflect not only differences in clinical practice, but also in the etiology of pneumonia and patterns of resistance in different regions of the world (Figure 4). Dr. File concluded by summarizing several measures which could reverse the impact of pneumonia. These include smoking cessation, pneumonoccal vaccines and controlling co-morbidities.
MRSA Deep Tissue Infections
Methicillin-resistant Staphylococcus aureus (MRSA) is increasingly implicated in deep tissue infections. In the US, an estimated two million Americans have been colonized with MRSA18. Dr. Moellering said that the proliferation of community-acquired MRSA (ca-MRSA) has been truly remarkable. In parts of the United States, 60% to 75% of all isolates of S. aureus are now methicillin-resistant. In Dr. Moellering's opinion, clinicians should have heightened suspicion for MRSA and a correspondingly low threshold for obtaining material for culture and susceptibility testing from community-acquired abscesses and other skin infections.
In settings with high incidence of ca-MRSA, Dr. Moellering advised that antibiotics with efficacy against MRSA should become the first-line treatment for community-acquired infections of skin and soft tissue. Typically, MRSA strains are resistant to all the beta-lactams, erythromycin, and ciprofloxacin. Many strains remain susceptible to gentamicin, cotrimoxazole, tetracyclines, rifampicin and fusidic acid19,20, and are almost invariably susceptible to vancomycin and teicoplanin. However, a few strains have been recently described which show a clinically significant reduction in susceptibility which may compromise the utility of these agents in clinical practice.
In reviewing the role of newer agents, Dr. Moellering highlighted that recent studies have shown linezolid to be more effective in eradicating MRSA than vancomycin in surgical-site infections (SSIs)21 and infective endocarditis22. A new study, out this year, demonstrated that daptomycin is effective in the treatment of bacteremia and endocarditis caused by S aureus 23. Tigecycline was demonstrated to be equivalent to vancomycin in recent phase III studies24, and was recently licensed for the treatment of complicated intra-abdominal, skin, and skin structure infections. Dr. Moellering concluded that these agents, and others currently in phase 3 development, offer promising alternatives to conventional therapy. That said, he emphasized that serious MRSA infections remain a major clinical and microbiological challenge.
- © 2006 MD Conference Express