Summary
Nintedanib, a tyrosine kinase inhibitor that targets fibroblast growth factor, platelet-derived growth factor, and vascular endothelial growth factor, was shown to lessen the decline in lung function and decrease acute exacerbations in patients with idiopathic pulmonary fibrosis (IPF) in the Phase 2 TOMORROW study [Richeldi L et al. N Engl J Med 2011]. This article presents the primary results for two 52-week Phase 3 randomized, placebo-controlled trials (Safety and Efficacy of BIBF 1120 at High Dose in Idiopathic Pulmonary Fibrosis Patients [INPULSIS] I and II) [Richeldi L et al. N Engl J Med 2014].
- Lower Respiratory Infections
- Pulmonary Clinical Trials
- Lower Respiratory Infections
- Pulmonary Clinical Trials
- Pulmonary & Critical Care
Nintedanib, a tyrosine kinase inhibitor that targets fibroblast growth factor, platelet-derived growth factor, and vascular endothelial growth factor, was shown to lessen the decline in lung function and decrease acute exacerbations in patients with idiopathic pulmonary fibrosis (IPF) in the Phase 2 TOMORROW study [Richeldi L et al. N Engl J Med 2011]. Luca Richedli, MD, PhD, University of Southampton, Southampton, United Kingdom, presented the primary results for two 52-week Phase 3 randomized, placebo-controlled trials (Safety and Efficacy of BIBF 1120 at High Dose in Idiopathic Pulmonary Fibrosis Patients [INPULSIS] I and II) [Richeldi L et al. N Engl J Med 2014].
In the INPULSIS trials, conducted at 205 sites in 24 countries globally, patients with IPF were randomly assigned in a 3:2 ratio to nintedanib 150 mg twice daily or placebo for 52 weeks. Key inclusion criteria were age ≥40 years, diagnosis of IPF within 5 years of randomization, chest high-resolution computed tomography (HRCT) performed within 12 months of screening, HRCT pattern and (if available) surgical lung biopsy pattern consistent with diagnosis of IPF as assessed centrally by 1 expert radiologist and 1 expert pathologist, forced vital capacity (FVC) ≥50% of predicted value, and carbon monoxide diffusing capacity 30% to 79% of predicted value. Key exclusion criteria included a ratio of forced expiratory volume in 1 second to FVC < 0.7 (prebronchodilator); treatment with N-acetylcysteine or prednisone >15 mg/day or equivalent within 2 weeks of screening; treatment with pirfenidone, azathioprine, cyclophosphamide, cyclosporine A, or any investigational drug within 8 weeks of screening; requirement for fibrinolysis, full-dose therapeutic anticoagulation, or high-dose antiplatelet therapy; and likelihood of undergoing lung transplantation during the study. The primary end point was the annual rate of decline in FVC. Key secondary end points were time to the first acute exacerbation and change in St. George's Respiratory Questionnaire total score over the 52 weeks.
Patient disposition is shown in Table 1. In total, 1066 patients were randomly assigned, with 25.2% and 17.6% medication discontinuation rates for nintedanib and placebo in INPULSIS-1 and 23.7% and 20.1% discontinuation rates, respectively, in INPULSIS-2 (Table 1).
Patient Disposition in the INPULSIS Trials*
Rates of study completion were similar, at 84.1% for nintedanib and 85.3% for placebo in INPULSUS-1 and 82.7% and 81.7%, respectively, in INPULSIS-2.
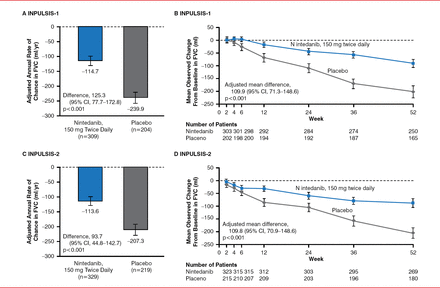
The primary end point was met in both trials, with significantly less decline of FVC in patients receiving nintedanib versus placebo (–114.7 vs −239.9 mL/year in INPULSIS-1 [p<0.001]; −113.6 vs −207.3 mL/year in INPULSIS-2 [p<0.0011]; Figure 1). Significance was maintained when the data were pooled (–113.6 vs −207.3 mL/year, p<0.0001). The trial arms were not significantly different in the time to first exacerbation for INPULSIS-1 alone (HR, 1.15; 95% CI, 0.54–2.42; p=0.67) or when the 2 trials were pooled (HR, 0.64; 95% CI, 0.39–1.05; p=0.08) but were significantly different in INPULSIS-2 (HR, 0.38; 95% CI, 0.19–0.77; p=0.005). A similar pattern was found for the respiratory questionnaire scores. Mortality was not significantly different between the treatment and placebo arms in the pooled data (5.5% for nintedanib and 7.8% for placebo; HR, 0.70; 95% CI, 0.43–1.12; p=0.14).
Primary Efficacy End Point
Reproduced with permission from L. Richeldi, MD, PhD.
Adverse event data are summarized in Table 2. The most frequent adverse events included diarrhea, nausea, cough, and bronchitis and were generally considered mild to moderate in severity. The rates of serious adverse events were similar between arms. However, it was noted that a higher proportion of patients in the nintedanib groups had elevated liver enzymes (4.9%–5.2% vs 0.5%– 0.9%) and myocardial infarctions (1.5%–1.6% vs 0.5%) compared with placebo.
Adverse Eventsa
Prof. Richeldi concluded that the INPULSIS-1 and INPULSIS-2 trials established the efficacy of nintedanib for minimizing the decline in lung function in patients with IPF and was generally well tolerated.
- © 2014 MD Conference Express®