Summary
This article provides an overview of the benefits of disruptive innovation in clinical research and in the development of cardiovascular medical therapeutics.
- Prevention & Screening
- Cardiology & Cardiovascular Medicine
- Prevention & Screening
Elliott M. Antman, MD, Harvard Medical School and the current president of the American Heart Association, provided an overview of the benefits of disruptive innovation in clinical research and in the development of cardiovascular medical therapeutics.
Disruptive innovation was a term coined to describe commercial innovation. It refers to a process where a new product or service that is better geared toward the target demand is introduced and it subsequently displaces established competing products or services that may be relatively too complex. In the nonmedical world, examples of disruptive innovation include e-mail, personal computers, and cell phones. Disruptive innovation in medical care could speed the transfer of beneficial therapeutic technologies from discovery to the bedside, according to Dr Antman.
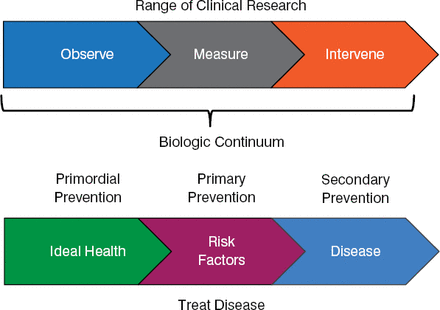
Traditional clinical research progresses from observation to measurement to active intervention across a biological continuum that involves disease prevention in healthy patients (eg, healthy diet), primary prevention of known risk factors, and secondary prevention in the treatment of disease (Figure 1).
Clinical Research Across the Biological Continuum
Reproduced with permission from EM Antman, MD
The range of cardiovascular technologies comprises 5 groups: drugs, devices, biologics (including antibodies and peptides), biomarker assays, and imaging techniques. The groups share similarities in their principles of clinical investigation. In the interest of time, Dr. Antman focused on drug treatment of atrial fibrillation (AF). AF is global, affecting a conservatively estimated 33.5 million people, predominantly the elderly. As the overall global population shifts to a higher proportion of elderly, the prevalence of AF is projected to increase.
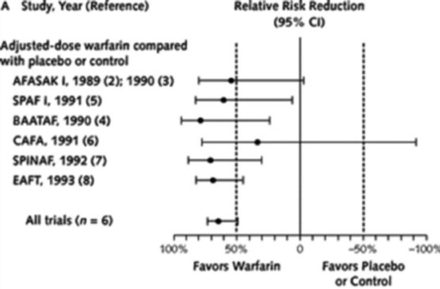
The current drug of choice is warfarin, a derivative of coumarin, which is a component of sweet clover that inhibits blood coagulation. Six seminal clinical trials conducted between 1989 and 1993 involving 2900 patients established warfarin as the standard of care. A meta-analysis of these trials reported an aggregated 64% reduction in total stroke and 67% reduction in ischemic stroke among patients receiving warfarin, compared with patients who were untreated or who received placebo (Figure 2) [Hart R et al. Ann Intern Med 2007].
Warfarin-Mediated Prevention of Stroke in Atrial Fibrillation
Reproduced from Hart RG et al. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. With permission from the American College of Physicians.
While effective, warfarin has several important shortcomings, including the need for monitoring and its interactions with other drugs and foods, which spurred efforts to find a replacement drug. Compounds with potential value include rivaroxaban, apixaban, edoxaban, and dabigatran. A recent meta-analysis of 4 clinical trials of these drugs involving > 70 000 patients reported similar activity as warfarin for ischemic stroke but a 50% reduction in the frequency of hemorrhagic stroke [Ruff CT et al. Lancet 2013]. Compliance in drug use is necessary, given the shorter half-lives of rivaroxaban, apixaban, edoxaban, and dabigatran compared with warfarin.
Suggested disruptive innovations that could reduce the high cost of drug development and help focus development include systems-based approaches, such as induced pluripotent stem cells (iPSCs) [Takahashi K et al. Cell 2007] and in vitro growth of specific tissue on a surface amenable to analysis (“organ on a chip”). Innovations that could increase the success of clinical development and the approval of identified drug candidates include adaptive designs and novel ways for doing the necessary research.
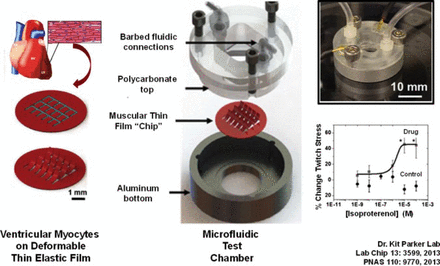
The classical assessment of medical therapeutics involves use of a patient population that is intended to be representative of the larger societal population that will be the target of treatment. Assessment can involve the use of subgroups. Rarely is assessment directed at the level of the individual. The systems-based approach is the opposite, with research focused on a “molecularly defined” individual used to develop therapeutics that can ultimately be used for populations. One example is the use of genotyping to identify the optimum dose of a drug or the disease-specific iPSCs that will be best metabolized by an individual [Mercola M et al. Circ Res 2013]. In another innovative approach, ventricular myocytes capable of contraction have been established on the surface of a plastic film that is positioned in a electrified chamber into which fluid can enter and exit. The design, termed a “heart on a chip” (Figure 3), can be used to examine the effects of different drugs and drug concentrations on cardiac cell activity [Agarwal A et al. Lab on a Chip 2013; McCain ML et al. PNAS 2013] and to model cardiac pathologies [Wang G et al. Nature Medicine 2014]. The system is also being used to explore the use of iPSCs in cardiac repair.
Tissue Engineering of a Heart on a Chip
Adapted from Agarwal A et al. Lab on a Chip 2013.
Another innovation could lie in clinical trial design—specifically, the use of a design that is more adaptive to emerging data. In this scenario, an ongoing study could be changed in response to new information. Adaptations could be made at the time of enrollment, during treatment (eg, alteration of dose or dose regimen), or during analyses of the data. This scenario is particularly apt to Phase 2 studies and can help better design them to yield results that will drive Phase 3 studies.
Yet another innovation allows the recording of a single-channel electrocardiogram through a modified smartphone via wireless transmission. Dr. Antman has personally diagnosed AF in a patient who transmitted data at the time that discomfort was occurring. Other wearable sensors can communicate with smartphones, with the information subsequently available to the global medical and research communities via the Internet. The Health eHeart Study underway by researchers at the University of California, San Francisco is monitoring the health of participants on the basis of data capture conducted via the Internet.
As a final example, Dr. Antman described the embedding of clinical research in clinical care, in which randomization established at the point of care (eg, between 2 drugs of proven benefit) would be recorded in the electronic medical record. The accumulated results of patient response would simultaneously provide clinical treatment and conduct a comparative clinical study of drug efficacy [Antman EM, Harrington RA. JAMA 2012]. Randomized clinical trials are valuable but capture information only for a defined time, even with decade-long follow-up. Randomization conducted among a free-living cohort, such as the Health eHeart Study, would complement the results of randomized clinical trials and could ultimately provide an “information commons” that is independent of trial length and that spans the biological continuum from health to clinical disease.
The convergence of biotechnology, sensor technology, and information technology (including mobile health) is beginning to enable clinical researchers and health providers to better address patient care.
- © 2014 MD Conference Express®