Summary
The Effective Anticoagulation With Factor Xa Next Generation in the Atrial Fibrillation—TIMI 48 trial [ENGAGE AF-TIMI 48; Giugliano RP et al. N Engl J Med. 2013] of 21?105 patients with atrial fibrillation and CHADS2 scores =?2 reported the noninferiority of the oral factor Xa inhibitor edoxaban 30 and 60 mg once daily versus warfarin and dose-related decreases in major bleeding. This article presents the results of a subanalysis showing that the protocol-driven dose adjustments in ENGAGE AF maintained the efficacy of edoxaban and reduced bleeding.
- Thrombotic Disorders
- Arrhythmias
- Cardiology Clinical Trials
- Cardiology
- Thrombotic Disorders
- Arrhythmias
- Cardiology Clinical Trials
The Effective Anticoagulation With Factor Xa Next Generation in the Atrial Fibrillation—TIMI 48 trial [ENGAGE AF-TIMI 48; Giugliano RP et al. N Engl J Med. 2013] of 21 105 patients with atrial fibrillation and CHADS2 scores ≥ 2 reported the noninferiority of the oral factor Xa inhibitor edoxaban 30 and 60 mg once daily versus warfarin and dose-related decreases in major bleeding. Christian T. Ruff, MD, MPH, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented the results of a subanalysis showing that the protocol-driven dose adjustments in ENGAGE AF maintained the efficacy of edoxaban and reduced bleeding.
By way of background, the novel oral anticoagulants (NOACs), such as edoxaban, unlike warfarin, seem to provide fixed dosing therapeutic anticoagulation without the need for routine monitoring. However, an emerging question is whether the drug concentration or anticoagulant activity should be measured to optimize the risk/benefit ratio of a NOAC. Therefore, this subanalysis correlated the trough plasma concentrations of edoxaban in 6780 study patients and the anti—factor Xa activity in 2865 of the 6780 patients, measured 1 month after randomization with the edoxaban dose. The efficacy and safety outcomes were compared in the no dose reduction (NDR) and dose reduction (DR) groups in the edoxaban arms against warfarin.
The primary efficacy outcome was stroke or systemic embolic events (SEEs), and the primary safety outcome was major bleeding defined by International Society on Thrombosis and Haemostasis criteria. At baseline, or if any factors developed during the trial, doses in both edoxaban arms were reduced by 50% for patients with reduced renal function (creatinine clearance 30–50 mL/min), those with lower weight (≤ 60 kg), and those coprescribed a potent P-glycoprotein inhibitor, which are indicators of increased bleeding risk or increased exposure to edoxaban.
The mean trough concentrations of edoxaban were 48.5 ± 45.8 and 24.5 ± 22.7 ng/mL with the 60- and 30-mg doses in the NDR group and 34.6 ± 30.9 and 16.0 ± 14.5 ng/mL in the DR groupo, respectively.
Mean trough anti—factor Xa activity was 0.85 ± 0.76 and 0.44 ± 0.37 IU/mL in the NDR group with the 60- and 30-mg doses and 0.64 ± 0.54 and 0.35 ± 0.28 IU/mL in the DR group, respectively. There was a good correlation between the trough edoxaban concentration and trough anti—factor Xa activity, irrespective of the edoxaban dose or DR (r = 0.96, P < .0001).
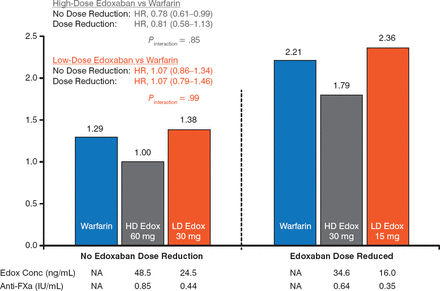
For the primary outcome, the efficacy of edoxaban (60 and 30 mg) versus warfarin was similar irrespective of a DR. Edoxaban 15 mg was associated with a nonsignificant increased risk versus warfarin and edoxaban 30 mg (Figure 1).
Primary Outcome With Maintained or Lowered High- and Low-Dose Edoxaban
Edox, edoxaban; FXa, factor Xa; HD, high-dose; LD, low-dose; NA, not applicable; SEE, systemic embolic event.
Reproduced with permission from C Ruff, MD, MPH.
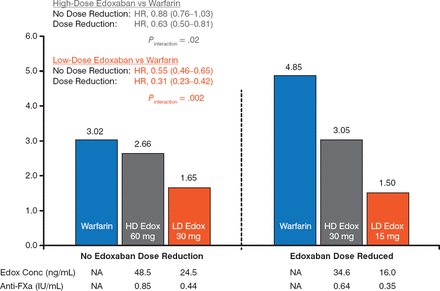
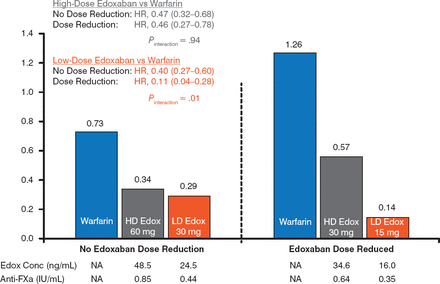
The risk for major bleeding was lower with both doses of edoxaban versus warfarin. For both doses, there was a significantly lower risk for major bleeding in the DR group versus the NDR group (P Interaction = .02 for e doxaban 60 mg, P Interaction = .002 for edoxaban 30 mg; Figure 2). A similar finding was evident for the annual risk for intracranial hemorrhage, with a significant reduction in the DR group versus the NDR group in the edoxaban 30 mg arm (Figure 3).
Annual Risk for Major Bleeding
Edox, edoxaban; FXa, factor Xa; HD, high-dose; LD, low-dose; NA, not applicable; SEE, systemic embolic event.
Reproduced with permission from C Ruff, MD, MPH.
Annual Risk for Intracranial Hemorrhage
Edox, edoxaban; FXa, factor Xa; HD, high-dose; LD, low-dose; NA, not applicable; SEE, systemic embolic event.
Reproduced with permission from C Ruff, MD, MPH.
The therapeutic window of edoxaban showed that the dose-response curve was steepest for major bleeding, shallower for stroke and SEEs, and nearly flat for intracranial hemorrhage.
The mean edoxaban exposure and anti—factor Xa activity was reduced by 29% to 35% and 20% to 25%, respectively, in the DR and NDR groups. These data validate the strategy of tailoring the dose of NOACs on the basis of clinical factors alone to achieve the dual goal of preventing excess drug levels and optimizing an individual patient's risk for ischemic and bleeding events and demonstrate that the therapeutic window for edoxaban is narrower for major bleeding than thromboembolism. Dose adjustment of edoxaban on the basis of clinical features obviates the need to measure drug levels or anticoagulant activity.
- © 2014 MD Conference Express®