Summary
This articls presented results from the Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia trial [ODYSSEY COMBO II; NCT01644188]. This phase 3 trial showed that for patients with high cholesterol and existing or increased risk of cardiovascular disease, alirocumab significantly improved cholesterol levels as compared to ezetimibe, when added to regular statin therapy.
- Cardiology
- Lipid Disorders
- Cardiology Clinical Trials
- Cardiology
- Lipid Disorders
- Cardiology Clinical Trials
Christopher P. Cannon, MD, Harvard Clinical Research Institute, Boston, Massachusetts, USA, presented results from the Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia trial [ODYSSEY COMBO II; NCT01644188]. This phase 3 trial showed that for patients with high cholesterol and existing or increased risk of cardiovascular disease (CVD), alirocumab significantly improved cholesterol levels as compared to ezetimibe, when added to regular statin therapy.
Over the last few decades, statins have been the mainstay of therapy for patients with high levels of low-density lipoprotein cholesterol (LDL-C). However, despite their significant clinical efficacy in most patients, a large residual risk remains for the development of atherosclerotic CVD [Baigent C et al. Lancet. 2010].
Proprotein convertase subtilisin kexin type 9 (PCSK9) inhibition represents a promising new strategy for the treatment of hypercholesterolemia that may complement statin therapy [Switzer MP et al. Cardiovasc Hematol Agents Med Chem. 2013]. Monoclonal antibodies (mAbs) against PCSK9 have recently been shown to be highly efficacious in lowering LDL-C, with a favorable adverse event profile in early clinical trials. Alirocumab is an investigational mAb that targets and blocks PCSK9 [Roth EM, Diller P. Future Cardiol. 2014].
ODYSSEY COMBO II is a double-blind, parallel-group multicenter study being conducted over 104 weeks to evaluate the safety and efficacy of alirocumab, compared with ezetimibe, among patients (n = 720) with hypercholesterolemia who are at high cardiovascular risk and who had inadequate LDL-C control at baseline despite stable maximally tolerated statin therapy.
Inclusion criteria included either a history of CVD and LDL-C ≥ 1.81 mmol/L (≥ 70 mg/dL) or no history of CVD but with other risk factors and LDL-C ≥ 2.59 mmol/L (≥ 100 mg/dL) and receipt of a maximally tolerated daily statin dose (stable for > 4 weeks prior to screening). Exclusion criteria included receipt of other lipid-lowering therapies [Colhoun HM et al. BMC Cardiovasc Disord. 2014].
Patients were randomized 2:1 to either alirocumab (75 mg, subcutaneously, Q2W; n = 479) or ezetimibe (10 mg, daily; n = 241) in addition to statin therapy. After 12 weeks, the dose of alirocumab was increased to 150 mg, Q2W, if the week 8 LDL-C level was ≥ 1.81 mmol/L (70 mg/dL). The primary end point was the percentage change in LDL-C from baseline to week 24.
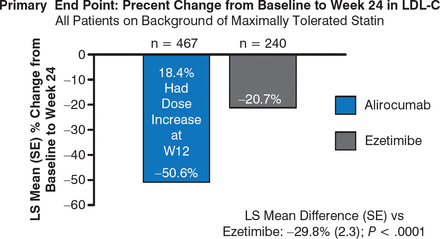
At week 24, alirocumab significantly reduced LDL-C levels as compared with ezetimibe (–50.6% vs −20.7%; P < .0001; Figure 1), with a least squares mean difference versus ezetimibe of −29.8% (P < .0001). This reduction was maintained to 52 weeks (–49.5% for alirocumab vs −18.3% for ezetimibe).
Effect of Alirocumab and Ezetimibe on LDL-C Levels at 24 Weeks
LDL-C, low-density lipoprotein cholesterol; LS, least squares (a statistical mean estimated from a linear model); SE, standard error.
Reproduced with permission from CP Cannon, MD.
At week 24, the majority of patients in the alirocumab arm were able to achieve a prespecified LDL-C target of < 1.81 mmol/L (70 mg/dL), with 77.0% and 45.6% of alirocumab and ezetimibe patients, respectively, achieving this level (P < .0001). In a post hoc analysis of a lower LDL-C target of < 1.3 mmol/L (50 mg/dL), 60.3% of alirocumab versus 14.2% of ezetimibe patients, respectively, achieved this lower level.
In an analysis of all safety data collected until last the patient visit at week 52, alirocumab appeared to be well tolerated. Treatment-emergent adverse events (TEAEs) occurred in 71.2% patients in the alirocumab arm and 67.2% of those in the ezetimibe arm and led to discontinuation in 7.5% and 5.4% patients in each treatment arm, respectively. The most commonly reported TEAEs (occurring in ≥ 5% of patients in each treatment arm) were upper respiratory tract infection, accidental overdose, dizziness, and myalgia.
Dr Cannon summarized that, in this population of patients with high CVD risk who were unable to achieve targets with maximal statin use, a treat-to-target approach with alirocumab (where ∼ 80% of patients did not need to up-titrate the initial dose) allowed more than three-quarters of patients to achieve target LDL-C levels at week 24.
- © 2014 MD Conference Express®