Summary
Treatment of drug-eluting stent in-stent restenosis (DES-ISR) is challenging. Results from the Restenosis Intra-Stent of Drug-Eluting Stents: Paclitaxel-Eluting Balloon vs Everolimus-Eluting Stent trial [RIBS IV; NCT01239940] indicate that DES provide superior late angiographic clinical outcomes compared with drug-eluting balloons.
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiology
Treatment of drug-eluting stent in-stent restenosis (DES-ISR) is challenging. Results from the Restenosis Intra-Stent of Drug-Eluting Stents: Paclitaxel-Eluting Balloon vs Everolimus-Eluting Stent trial [RIBS IV; NCT01239940], presented by Fernando Alfonso, MD, PhD, Hospital Universitario “La Princesa,” Madrid, Spain, indicate that DES provide superior late angiographic clinical outcomes compared with drug-eluting balloons (DEBs).
The objective of this multicenter (23 sites in Spain), prospective, randomized phase 4 study was to compare the efficacy of a paclitaxel DEB with an everolimus-eluting stent (EES) in patients with DES-ISR. The study included patients (n = 309; mean age, 66 years) with DES-ISR > 50% stenosis, angina or silent ischemia, and ISR suitable for conventional balloon angioplasty and stenting were randomized to EES (n = 155) or DEB (n = 154). Patients with an undefined stent location, ISR < 1 month, vessel diameter < 2 mm, ISR length > 30 mm, or ISR outside the stent were excluded. The primary end point was minimal lumen diameter (MLD) at late angiographic follow up (6–9 months) based on quantitative coronary angiography. Secondary outcome measures were a composite of clinical (including cardiac death, myocardial infarction [MI], and revascularization), and angiographic (eg, percentage of diameter stenosis, angiographic late lumen loss, and binary restenosis) end points at 6 to 9 months and 1 and 3 years. The design of this study has been published [Alfonso F et al. EuroIntervention. 2014].
Approximately 49% of participants had a history of MI and 11% had prior bypass surgery. Unstable angina was present in 52% of subjects; stable angina in 48%. Mean left ventricular ejection fraction (LVEF) was 58%. The mean number of diseased vessels was 1.65 with the most common (48%) target vessel being the left anterior descending coronary artery. Mean length of initial stent was 21 ± 7 mm and maximal pressure was 16 ± 3 atm. Mean time to ISR was 547 days.
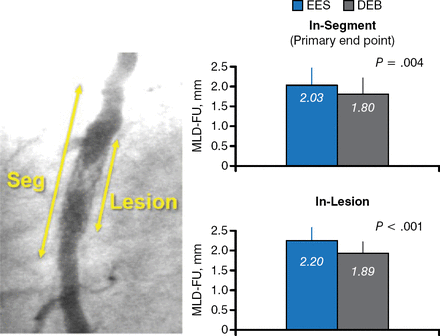
At 9 months, in-segment MLD was larger with EES compared with DEB (2.03 vs 1.80, respectively; P = .004). In-lesion MLD was also larger (2.20 vs 1.89 EES and DEB, respectively; P < .001; Figure 1) as were the cumulative frequency distribution curves for in-segment MLD (P = .004 at 9 months; P = .04 post procedure). Further analysis of MLD using 10 prespecified clinical and angiographic variables revealed consistent results favoring EES. Trends favored EES for both binary restenosis and late lumen loss.
In-Segment and In-Lesion MLD Larger With EES Compared With DEB
DEB, drug-eluting balloon; EES, everolimus-eluting stent.
Reproduced with permission from F Alfonso, MD, PhD.
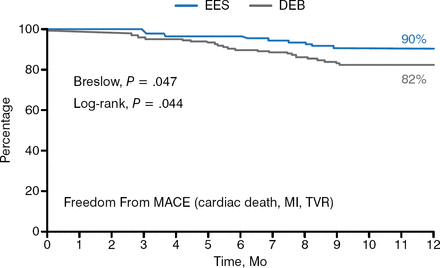
After 1 year, subjects receiving an EES had higher rates of freedom from target lesion revascularization (96% vs 87%; P = .008) and freedom from major adverse coronary events, including cardiac death, MI, and target vessel revascularization (90% vs 82%; P = .044; Figure 2). There were 1 definite, 1 probable, and 2 possible thromboses in the EES arm and 2 definitive, 1 probable, and 1 possible thrombosis in the DEB arm. Five patients in the DEB group crossed over to stenting.
MACE Outcomes at 1 Year in EES and DEB Groups
DEB, drug-eluting balloon; EES, everolimus-eluting stent, MACE, major cardiac adverse events.
Reproduced with permission from F Alfonso, MD, PhD.
In this first randomized trial of EES vs DEB in patients with DES-ISR, EES provided superior late angiographic results than DEB. In these patients EES also provided better late clinical results, driven by a significant reduction in the rate of target vessel revascularization. Further studies with more patients and longer follow-up are warranted in this setting.
- © 2014 MD Conference Express®