Summary
In the Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation trial [ISAR-TRIPLE; NCT00776633], 6 weeks of triple therapy including the oral antiplatelet compound clopidogrel is not superior to a 6-month regimen following implantation of a drug-eluting stent.
- Interventional Techniques & Devices
- Thrombotic Disorders
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Thrombotic Disorders
- Cardiology
- Cardiology Clinical Trials
In the Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation trial [ISAR-TRIPLE; NCT00776633], 6 weeks of triple therapy including the oral antiplatelet compound clopidogrel is not superior to a 6-month regimen following implantation of a drug-eluting stent (DES). The results were reported by Nikolaus Sarafoff, MD, Technische Universität München, Munich, Germany.
Previous studies support the view that combined oral anticoagulant/antiplatelet therapy is advantageous in reducing adverse outcomes following cardiac surgeries [Connolly S et al. Lancet. 2006]. However, triple therapy comes with the drawback of increased risk of bleeding. The optimal length of therapy to maximize the benefits while minimizing bleeding risk following DES implantation is unknown.
The risk of stent thrombosis is greatest soon after percutaneous coronary intervention and declines thereafter. Also, bleeding risk depends on the length and intensity of oral anticoagulant therapy [Lip GYH et al. Eur Heart J. 2014]. Informed by this knowledge, the ISAR-TRIPLE prospective, randomized, open-label trial evaluated clinical outcomes of a 6-week and 6-month regimen of clopidogrel along with aspirin and oral anticoagulation following DES implantation in 614 patients [Fiedler KA et al. Am Heart J. 2014]. The hypothesis was that the shorter course of therapy is superior.
DES implantation and need for oral anticoagulation were the inclusion criteria. Exclusion criteria included prior stent thrombosis and DES implantation in the left main coronary artery. The primary end point was death, myocardial infarction (MI), confirmed stent thrombosis, stroke, or major bleeding at 9 months. Secondary end points included ischemic complications (cardiac death, MI, stent thrombosis, ischemic stroke) and major bleeding.
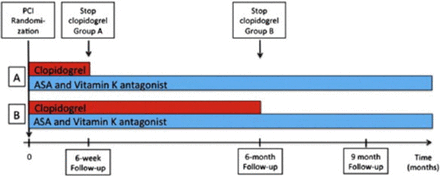
All patients received aspirin (75–200 mg QD) and vitamin K antagonist along with clopidogrel 75 mg QD for 6 weeks (n = 307) or 6 months (n = 307). Phenprocoumon or warfarin were used in patients with mechanical valves (5% and 9% of the 6-week and 6-month group, respectively) to achieve a target internal normalized ratio. Clinical follow-up was done after 9 months for most patients (n = 606 [98.7%]; Figure 1).
ISAR-TRIPLE Design
ASA, aspirin; PCI, percutaneous coronary intervention.
A: 6-week group; B: 6-month group.
Reproduced from American Heart Journal, 167, Fiedler KA et al, Rationale and design of The Intracoronary Stenting and Antithrombotic Regimen—Testing of a six-week versus a six-month clopidogrel treatment Regimen In Patients with concomitant aspirin and oraL anticoagulant therapy following drug-Eluting stenting (ISAR-TRIPLE) study, 459–465, Copyright 2014, with permission from Elsevier.
At baseline, the 2 groups were similar in age; sex; history of MI; prevalence of diabetes, acute coronary syndrome, and stable angina; and indication of oral anticoagulants. Compliance with all medications was excellent, with the exceptions of clopidogrel use by those in the 6-week group after 6 weeks (26% vs 87% in the 6-month group; P < .001) and at the 9-month follow-up (23% vs 35%; P < .001).
There were no significant differences between the 2 groups in either the primary end point (HR, 1.14; 95% CI, 0.68 to 1.91; P = .63) or the secondary end points of ischemic complications (HR, 0.93; 95% CI, 0.43 to 2.05; P = .87) and major bleeding (HR, 1.35; 95% CI, 0.64 to 2.84; P = .44).
Parsing out the secondary end point data did reveal a significant difference in MI (6-week, n = 6 [2.0%]; 6-month, n = 0; P = .029). Bleeding Academic Research Consortium (BARC)-defined bleeding overall did not differ significantly between the groups (6-week, 37.6%; 6-month, 40.2%; HR, 0.94; 95% CI, 0.73 to 1.21; P = .63). But, comparison of BARC-defined bleeding prior to randomization with that occurring at 9 months was significant (6-week, 20.5%; 6-month, 27.9%; HR, 0.68; 95% CI, 0.47 to 0.98; P = .04).
Prof Sarafoff concluded that shortening clopidogrel therapy from 6 months to 6 weeks after DES implantation in patients who are also receiving aspirin and oral anticoagulation is not superior in terms of net clinical outcomes.
- © 2014 MD Conference Express®