Summary
Patient adherence to glucose-lowering medications is a major challenge in type 2 diabetes mellitus. It is thought that an effective, well-tolerated, weekly oral antihyperglycemic agent could provide better glycemic control due to increased adherence. This article examines results of the MK-3102 Phase III Clinical Trial [NCT01703221], which evaluated the safety and efficacy of the novel dipeptidyl peptidase-4 inhibitor omarigliptin compared with placebo and sitagliptin in Japanese patients with type 2 diabetes mellitus.
- Diabetes Mellitus
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
Ira Gantz, MD, Merck Sharp & Dohme, Whitehouse Station, New Jersey, USA, presented the results of the MK-3102 Phase III Clinical Trial [NCT01703221; Gantz I et al. EASD 2014 (Oral Presentation 115)], a double-blind, parallel-group, active- and placebo-controlled trial to evaluate the safety and efficacy of the novel dipeptidyl peptidase-4 (DPP-4) inhibitor omarigliptin compared with placebo and sitagliptin in Japanese patients with type 2 diabetes mellitus (T2DM). This trial was the first phase 3 study results presented for this drug and showed that once-weekly omarigliptin was not inferior to sitagliptin in lowering HbA1c levels and had a similar safety profile when compared with sitagliptin.
Patient adherence to glucose-lowering medications is a major challenge in T2DM. It is thought that an effective, well-tolerated, weekly oral antihyperglycemic agent could provide better glycemic control due to increased adherence. Once-weekly incretin-based therapies in injectable formulations have become available, but to date no oral formulations have emerged.
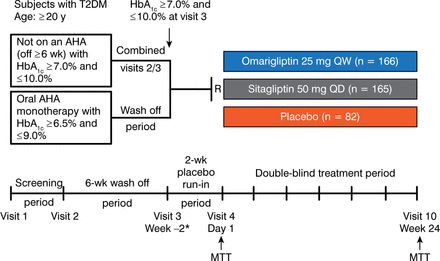
This phase 3, double-blind, noninferiority trial assessed the efficacy, safety, and tolerability of omarigliptin 25 mg weekly compared with sitagliptin 50 mg once daily, and compared with placebo. The study compared a 25-mg/wk dose of omarigliptin vs the standard 50-mg/d starting sitagliptin dose in patients in Japan. The primary efficacy end point was the change in HbA1c levels from baseline to week 24. The study design is shown in detail in Figure 1.
Omarigliptin vs Sitagliptin Phase 3 Study Design
AHA, antihyperglycemic agent; MTT, mixed-meal tolerance test; R, randomization; T2DM, type 2 diabetes mellitus.
MTT at visit 10 performed 1 d after sitagliptin or 7 d after omarigliptin.
Reproduced with permission from I Gantz, MD.
The study population included Japanese adults (aged ≥ 20 years) with T2DM who were randomly assigned to 1 of 3 treatment groups: once-weekly omarigliptin 25 mg (n = 166), once-daily sitagliptin 50 mg (n = 165), or placebo (n = 82). A mixed-meal tolerance test was performed the day before administering the study drug and again at 24 weeks, 1 day after the last dose of sitagliptin or 7 days after the last dose of omarigliptin. At baseline, randomized patients had a mean HbA1c concentration of 7.9%, 8.0%, and 8.1% in the omarigliptin, sitagliptin, and placebo groups, respectively. Mean fasting plasma glucose levels were also similar among treatment groups.
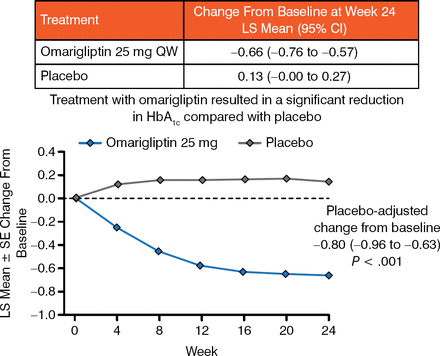
At week 24, omarigliptin significantly reduced HbA1c levels by −0.66% from baseline; placebo-adjusted change from baseline in HbA1c was −0.80% (P < .001; Figure 2).
Change in HbA1c (%) Through Week 24
LS, least squares; SE standard error.
Reproduced with permission from I Gantz, MD.
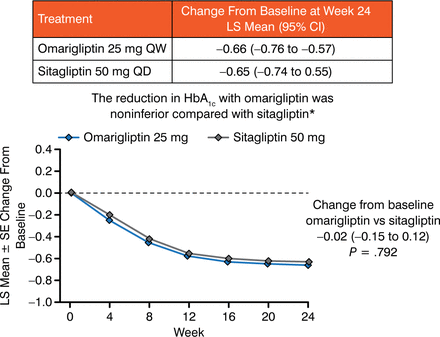
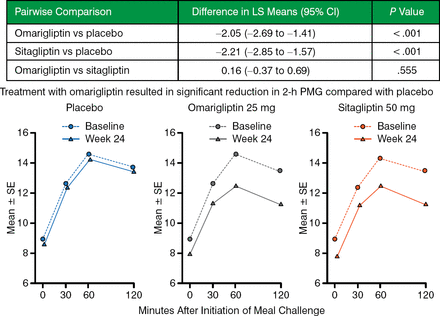
The change relative to sitagliptin was −0.02% (Figure 3) and met the prespecified noninferiority criterion. Compared with placebo, omarigliptin significantly reduced 2-hour postmeal glucose and fasting plasma glucose (Figure 4).
Change in HbA1c (%) Through Week 24
LS, least squares; SE standard error.
Reproduced with permission from I Gantz, MD.
Change in 2-Hour Postmeal Glucose at Week 24
LS, least squares; PMG, postmeal glucose; SE standard error.
Reproduced with permission from I Gantz, MD.
Omarigliptin was well tolerated, and there were no episodes of symptomatic or severe hypoglycemia. There were also no meaningful changes in weight, laboratory safety measures, heart rate, blood pressure, or electrocardiogram (ECG) intervals (including QTc), and no reports of pancreatitis in any treatment group. There were no meaningful differences in the incidences of adverse events with omarigliptin compared with placebo and sitagliptin (Table 1).
Specific AEs With an Incidence ≥ 3% in One or More Treatment Group by System Organ Class
In conclusion, in Japanese patients with T2DM, treatment with once-weekly omarigliptin over 24 weeks achieved statistically significant, clinically meaningful improvements in glycemic control vs placebo and was generally well tolerated. In this trial, the glycemic efficacy and safety profile of once-weekly omarigliptin were similar to those of once-daily sitagliptin.
- © 2014 MD Conference Express®