Summary
Combined empagliflozin and linagliptin as add-on therapy to metformin provided enhanced glucose-reducing efficacy when compared with either drug as monotherapy in individuals with type 2 diabetes mellitus, as discussed in this article.
- Hyperglycemia/Hypoglycemia
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
Combined empagliflozin and linagliptin as add-on therapy to metformin provided enhanced glucose-reducing efficacy when compared with either drug as monotherapy in individuals with type 2 diabetes mellitus (T2DM), stated Ralph A. DeFronzo, MD, University of Texas, San Antonio, Texas, USA, while presenting results from a phase 3 double-blind, parallel-group study.
According to Dr DeFronzo, sodium-glucose cotransporter 2 inhibitors, such as empagliflozin, are a new class of drugs for the management of T2DM. Linagliptin is a dipeptidyl peptidase 4 inhibitor and is considered to provide additional benefit in T2DM treatment when combined with empagliflozin, due to its ability to inhibit glucagon and enhance insulin secretion.
This study aimed to investigate the efficacy and safety of empagliflozin and linagliptin, individually and in combination as add-on therapy to metformin in adults with T2DM.
Patients in this study were all > 18 years of age, with a body mass index of ≤ 45 kg/m2 and HbA1c levels between 7% and 10.5%. All subjects had to have received a stable background therapy of metformin for ≥ 12 weeks before randomization. Patients were excluded who had an estimated glomerular filtration rate < 60 mL/min/1.73 m2 during screening or placebo run-in.
Dr DeFronzo and colleagues randomized 677 patients to empagliflozin-25 mg + linagliptin-5 mg (n = 137), empagliflozin-10 mg + linagliptin-5 mg (n = 136), empagliflozin-25 mg (n = 135), empagliflozin-10 mg (n = 134), and linagliptin-5 mg (n = 135). The primary end point was the change from baseline in HbA1c at week 24. The study continued to week 52.
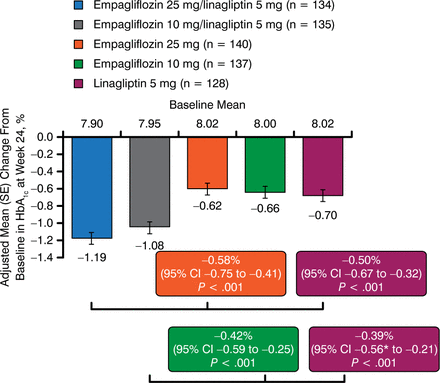
At 24 weeks, patients treated with both fixed-dose combinations of empagliflozin + linagliptin showed significant HbA1c reductions as compared with the monotherapies, with a higher percentage of the combinations achieving HbA1c < 7% (P < .001; Figure 1). Adjusted mean HbA1c changes from baseline for those receiving the combination therapies were −1.19% for empagliflozin −25 mg + linagliptin-5 mg and −1.08% for empagliflozin −10 mg + linagliptin-5 mg. For those receiving one of the monotherapies, the changes observed were −0.62% for empagliflozin-25 mg, −0.66% for empagliflozin −10 mg, and −0.70% for linagliptin-5 mg [DeFronzo R et al. Diabetes. 2014 (abstr 130-LB)].
Change in Mean HbA1c From Baseline at 24 Weeks
SE, standard error.
Source: DeFronzo R et al. Diabetes. 2014 (abstr 130-LB).
This effect continued to 52 weeks, at which point significantly higher percentages of patients treated with both fixed-dose combinations continued to show reductions in HbA1c < 7% as compared with the monotherapies (P < .001). Adjusted mean HbA1c changes from baseline for the combinations at 52 weeks were −1.21% for empagliflozin −25 mg + linagliptin-5 mg and −1.05% for empagliflozin −10 mg + linagliptin-5 mg. The individual monotherapies showed changes of −0.64% for empagliflozin −25 mg, −0.69% for empagliflozin-10 mg, and −0.48% for linagliptin-5 mg. While the combinations demonstrated efficacy beyond that of the individual monotherapies, Dr DeFronzo noted that the effects were “not completely additive.”
Empagliflozin monotherapy and the fixed-dose combinations reduced systolic blood pressure from baseline by a range of 2.8 to 3.6 mm Hg. Combination therapy with empagliflozin-25 mg + linagliptin-5 mg and empagliflozin −10 mg + linagliptin-5 mg significantly reduced systolic blood pressure when compared with linagliptin −5 mg monotherapy (P = .005 and P = .02, respectively).
Combined empagliflozin and linagliptin was well tolerated, and the safety profile was similar to that demonstrated by the individual components. The incidence of hypoglycemia was similar in all 5 groups (between 2% and 5%), as was the incidence of adverse events leading to treatment discontinuation (between 2% and 9%), urinary tract infection (between 14% and 20%), and genital infection (between 3% and 12%).
In his concluding remarks, Dr DeFronzo said that these results show that combination therapies are more effective in reducing HbA1c versus monotherapy with the same drugs.
- © 2014 MD Conference Express®