Summary
Hyaluronic acid (HA) is a naturally occurring biological substance that has proven safe to use for treating plantar fasciitis. The purpose of this prospective comparative study was to determine the efficacy of HA on plantar fasciitis that did not respond to common noninvasive treatment methods, as discussed in this article.
- Foot & Ankle Conditions
- Orthopaedics Clinical Trials
- Foot & Ankle Conditions
- Orthopaedics Clinical Trials
- Orthopaedics
Ultrasound-guided hyaluronic acid (HA) injection and dry needling are effective and safe for patients with plantar fasciitis who did not respond to commonly performed conservative therapy, according to Kang Lee, MD, Kangwon National University Hospital, Chuncheon, Republic of Korea.
HA is a naturally occurring biological substance that has proven safe to use for treating plantar fasciitis. The purpose of this prospective comparative study was to determine the efficacy of HA on plantar fasciitis that did not respond to common noninvasive treatment methods. Patients with pain in both heels for ≥ 10 months whose symptoms were not relieved by or who experienced recurrence after conservative treatment were enrolled. Plantar fasciitis was diagnosed as first-step pain, pain after < 40 minutes of walking, tenderness, and thickened plantar fascia on ultrasound.
Of the 212 patients eligible for assessment, 81 were enrolled to receive HA on their right foot and dry needling on the left. Twenty patients were lost to follow-up, leaving 61 patients for the final analysis. Patients had a mean age of 46 years (more women than men), a mean body mass index of 29.4 kg/m2, and a mean duration of symptoms of 14.9 months. Various treatment modalities were tried before enrollment, including stretching, physiotherapy, oral medications, steroid injections, and acupuncture.
Prior to treatment, patients received a prefabricated insole and plantar fascia-specific stretching education (3 minutes twice daily). Following injection of 1 ml of 1% lidocaine in each foot, 2 ml (20 mg) of sodium hyaluronate (3000 kDa/ml) was injected into the right foot under ultrasound guidance into 3 areas: the insertion of the plantar fascia to the calcaneus, the fascia itself, and the perifascial space. Dry needling was performed on the left foot. Both treatments were administered once per week for 3 weeks. Patients were assessed for pain every 2 months after injection using the visual analog scale (VAS) and the American Orthopaedic Foot & Ankle Society (AOFAS) Ankle-Hindfoot Scale. Plantar fascia thickness was assessed at 2 and 6 months.
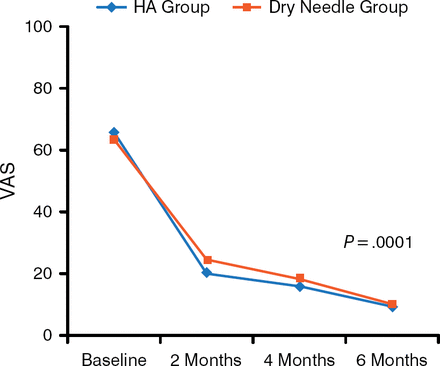
Pain assessment with VAS showed a significant improvement compared with baseline in both groups (P = .0001). Patient-rated pain scores dropped from a mean of 65 for the HA group and 63 for the dry needle group at baseline to 19 and 24, respectively, at 2 months, and 9 and 9.7, respectively, at 6 months (Figure 1).
Mean VAS Decreases Following HA and Dry Needling
HA, hyaluronic acid; VAS, visual analog scale.
Reproduced with permission from K Lee, MD.
A significant difference between the HA and dry-needling arms for VAS was noted at 2 months (P = .039), but not at 4 or 6 months. The AOFAS score increased from 55.1 ± 13.9 to 84.0 ± 6.5 in the HA group and from 55.3 ± 12.7 to 83.8 ± 6.7 in the dry-needling group. The between-group difference was not significant. The thickness of the plantar fascia between baseline and follow-up in both groups was not significantly different, nor was it significantly different between the 2 arms. There were no major complications. Injection site pain was experienced by 18 patients, and tingling sensation by 6 patients; both conditions spontaneously resolved.
In addition to its direct therapeutic effect, HA acts as a scaffold with internal bleeding from multiple punctures, which can promote healing. With regard to dry needling, the blood clot from multiple punctures may have stimulated the healing process, or the needle itself may have had some role. Additional studies are needed in this area.
These findings are limited because this was not a randomized study and had a short follow-up period. In addition, the initial treatment varied from patient to patient, and there may have been varied effectiveness among the 3 injection target points.
Nevertheless, this first prospective comparative study with HA for management of plantar fasciitis showed that HA is clinically effective and safe. Furthermore, the clinical course during treatment may have been altered by introduction of the needle itself into the plantar fascia, the authors concluded, regardless of the use of the injection.
- © 2014 MD Conference Express®