Summary
Presenters at the 2014 annual meeting of the American Society of Anesthesiologists discussed the guidelines on blood transfusion, the best practice regarding use of antiplatelet agents in a preoperative setting, and the use of factor concentrates in controlling perioperative bleeding.
- Cardiac Anesthesia
- Transfusion Medicine
- Anesthesiology Guidelines
- Hematology Guidelines
- Cardiac Anesthesia
- Transfusion Medicine
- Anesthesiology Guidelines
- Hematology Guidelines
- Anesthesiology
Presenters at the 2014 annual meeting of the American Society of Anesthesiologists discussed the guidelines on blood transfusion, the best practice regarding use of antiplatelet agents in a preoperative setting, and the use of factor concentrates in controlling perioperative bleeding.
TRANSFUSION GUIDELINES
Guidelines for when to provide blood transfusions contain large gray areas that require anesthesiologists to exercise good judgment when deciding whether to conduct a transfusion of whole blood for a given patient. Mark Ereth, MD, Mayo Clinic College of Medicine, Rochester, Minnesota, USA, reviewed considerations that influence transfusion decisions.
Common to guidelines available from blood banks and professional medical societies, including the American Society of Anesthesiologists, are recommendations to transfuse at hemoglobin (Hb) levels < 6 g/dL but not for Hb > 10 g/dL. Although helpful, this guideline leaves a large range of Hb concentration in the middle and for which there is no specific recommendation. Dr Ereth also reviewed the recommendations from the American Association of Blood Banks for hospitalized but hemodynamically stable patients [Carson JL et al. Ann Intern Med. 2012]. For patients in the intensive care unit, a strong recommendation was given for transfusion at Hb ≤ 7 g/dL, said to be backed by a high quality of evidence. Postoperatively, the recommendation for transfusion, again rated “strong” with “high quality of evidence,” moved up to Hb ≤ 8 g/dL when blood loss was expected or for such symptoms as chest pain, tachycardia, and lack of response to fluids. However, recommendations for transfusions for merely the presence of cardiovascular disease at Hb ≤ 8 g/dL or for observed symptoms were listed as “uncertain” and given a very low quality of evidence, while a recommendation to be guided by symptoms and Hb level for all patients was considered “weak,” again with a low quality of evidence.
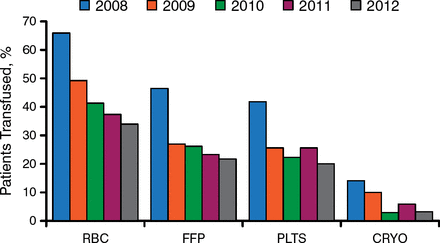
Dr Ereth said that when Hb is between 6 and 10 g/dL, anesthesiologists must evaluate the entire patient—including trauma and resuscitation status, volume status, and whether the patient is in shock or is actively bleeding—as well as trends in hematocrit levels. Overall, use of guidelines over the past several years has reduced the number transfusions considerably (Figure 1).
Reduction in Percentage of Transfusions for Cardiac Surgery
CRYO, cryoprecipitate; FFP, fresh frozen plasma; PLTS, platelet; RBC, red blood cell.
Reproduced with permission from M Ereth, MD.
PLATELET INHIBITORS
The history of cardiovascular medicine has depended on the use of antiplatelet agents and anticoagulants. Arterial clots formed by platelet–fibrinogen interactions are not completely blocked by use of heparin; venous clots and other venous thromboembolic phenomena are prevented by use of thrombin inhibitors [Tanaka KA et al. Anesth Analg. 2009]. Jerrold Levy MD, Duke University School of Medicine, Durham, North Carolina, USA, discussed current best practice in use of antiplatelet agents in a perioperative setting, focusing on some of the newer agents. It is well established that perioperative myocardial infarction increases risk of death after coronary artery bypass graft surgery [Gavard JA et al. J Thorac Cardiovasc Surg. 2003]. In a study comparing all–cause mortality from use of the newer prasugrel with that from clopidogrel (the more established antiplatelet agent), the prasugrel group had a significantly lower mortality of 2.3% compared with 8.7% in the clopidogrel group (P = .025) [Smith P et al. J Am Coll Cardiol. 2012].
Dr Levy compared the various agents based on the performance listed in their package inserts. Clopidogrel reaches an inhibition of platelet aggregation (IPA) level of 38% at 2 hours, with 16% of patients achieving a > 70% IPA level by 2 hours, with a maximum IPA level of 58% and a return to platelet function in 7 to 10 days [Gurbel PA et al. Circulation. 2009]. With prasugrel, 90% of patients are at a ≥ 50% IPA level by 1 hour following a 60–mg loading dose, with a maximum IPA level of 80% and a return of platelet function in 5 to 9 days. Ticagrelor delivers an 88% IPA level at 2 hours, with 90% of patients achieving > 70% IPA by 2 hours and with a maximum IPA level of 90% and a return to platelet function in 5 to 7 days [Gurbel PA et al. Circulation. 2009].
Regarding when it is safe to operate after antiplatelet therapy is discontinued, practice guidelines of the Society of Thoracic Surgeons call for stopping clopidogrel 5 days before surgery, but Dr Levy said that anesthesiologists might want to consider supplementing with point–of–care testing, as clopidogrel provides variability of effects in about 30% of patients. Newer agents, such as prasugrel and ticagrelor, are more predictable. Prasugrel should be stopped 7 days before surgery, and ticagrelor should be stopped 3 to 5 days before surgery.
FACTOR CONCENTRATES
As important as antiplatelet agents are in preventing the formation of clots, preventing perioperative bleeding is the concern at other times. The use of factor concentrates in controlling perioperative bleeding was explored by David Mazer, MD, St Michael's Hospital, Toronto, Canada. After providing a brief review of hemostasis, Dr Mazer indicated the different levels on the coagulation pathway where either prothrombin complex concentrates (PCCs) or fibrinogen exerts their action [Levy J et al. Anesth Analg. 2011].
PCCs contain vitamin K–dependent clotting proteins; they have the advantage of lyophilization, and they can be stored at room temperature for several years. No thawing or blood–type matching is required, and they can be rapidly administered without risk of fluid overload. The risk of viral transmission is negligible, but there is an increased cost when compared with fresh frozen plasma, and there is a small possibility of thromboembolic complications. Dr Mazer compared the PCCs commercially available in the United States and Canada, detailing their composition. All 4 factor PCCs contain factor II, factor VII, factor IX, and factor X, along with protein C, protein S, and heparin or antithrombin III. The PCC dose depends on the amount of clotting observed, from a low of 25 IU/kg to a high of 50 IU/kg. The duration of action is 6 to 8 hours, so vitamin K should be given for synthesis of clotting factors at 4 to 6 hours. Dr Mazer emphasized that despite their utility, potential side effects of PCCs include thromboemboli, viral transmission, and antibody production.
Guidelines from the European Society of Anaesthesiology (ESA) were also reviewed [Kozek–Langenecker SA et al. Eur J Anaesthesiol. 2013]. They promote use of coagulation factor concentrates, as these factors may reduce costs associated with transfusion in cardiac surgery, trauma, and liver transplants. The ESA further recommended that fibrinogen concentrate infusion be guided by point–of–care viscoelastic coagulation monitoring to best reduce perioperative blood loss in cardiovascular surgery. The ESA also suggested that anesthesiologists consider prophylactic preoperative infusion of fibrinogen concentrate in patients with low fibrinogen, as this may reduce bleeding after elective coronary artery bypass graft surgery. Regarding replacing blood products altogether, Dr Mazer cautioned that there are limited efficacy data available for concentrates and few good head–to–head comparisons of concentrates with nonconcentrate comparators. Furthermore, there are concerns about the risk of persistent thrombosis because the trials conducted have not been large enough to categorically declare that the products are safe. Finally, concentrates are likely to be costlier than the blood products.
- © 2014 MD Conference Express®