Summary
Heart failure phenotypes are currently categorized in various ways (eg, as acute vs chronic disease) or by the disease consequences. This article explores the role of some promising new biomarkers as novel approaches to define and treat acute heart failure phenotypes.
- Heart Failure Genomics
- Cardiology
- Heart Failure
- Cardiology Genomics
According to W. Frank Peacock, MD, Baylor College of Medicine, Houston, Texas, USA, a phenotypic division of a condition requires 3 features to be clinically useful:
-
It must identify a patient population with unique characteristics.
-
Its unique characteristics must manifest in a treatment response.
-
Predicted outcomes in the patient population must be able to be changed by intervention.
Although heart failure (HF) phenotypes are currently categorized in various ways (eg, as acute vs chronic disease) or by the disease consequences, Dr Peacock stressed that, in his opinion, these methods are unsatisfactory. In a symposium on HF, he joined a panel of speakers who explored the role of some promising new biomarkers as novel approaches to define and treat acute heart failure (AHF) phenotypes.
Rudolf A. de Boer, MD, University Medical Center, Groningen, The Netherlands, introduced the session with a discussion on galectin-3, an emerging prognostic biomarker in AHF. This bioactive protein is activated in cases of myocardial fibrosis. In the failing heart, it is secreted by macrophages at sites of tissue injury, where it stimulates the release of cytokines that promote fibro-blast differentiation into myofibroblasts [McCullough P et al. Rev Cardiovasc Med. 2011], a key step in cardiac remodeling after cardiac injury.
According to Prof de Boer, several studies have demonstrated that an elevated concentration of galectin-3 predicts all-cause mortality in the general population [deBoer RA et al. J Intern Med. 2012; Ho Je et al. J Am Coll Cardiol. 2012; Hillege HL et al. J Intern Med. 2001] and new-onset HF [Ginsberg E et al. J Am Coll Cardiol. 2014 (poster 1114–175); Ho Je et al. J Am Coll Cardiol. 2012]. It has also been shown to correlate with echocardiographic measures of left ventricular (LV) diastolic dysfunction [Seliger SL et al. J Am Coll Cardiol. 2014 (poster 1114–173)], and to predict outcomes in patients with HF with preserved LV ejection fraction [de Boer RA et al. Ann Med. 2011].
Galectin-3 appears to predict short-term outcomes after AHF. In a pooled analysis of 3 AHF studies, elevated galectin-3 was associated with a 2- to 3-fold increased risk of rehospitalization or death within 30 to 120 days of discharge [Meijers WC et al. Am Heart J. 2014]. Its prognostic value with respect to longer-term outcomes still remains less clear, however [Gullestad L et al. Am Heart J. 2012].
In the CORONA trial [Gullestad L et al. Eur Heart J. 2012], which examined the effect of galectin-3 on response to statin therapy, data showed that patients with a galactin-3 level < 19 ng/mL appeared to experience benefit from rosuvastatin therapy, compared with those with higher galactin-3 levels who did not benefit.
Prof de Boer noted that galectin-3 may also be useful in predicting absence of heart disease, and that possibly the most interesting application of galectin-3 may be targeted therapy, because experimental evidence suggests that galectin-3 inhibitors may ultimately be useful for the treatment of HF.
According to Allan S. Jaffe, MD, Mayo Clinic and Medical School, Rochester, Minnesota, USA, ST2 is a biomarker of cardiomyocyte stress that has proven to be a useful predictor of mortality in HF and myocardial infarction (MI). It is a member of the interleukin (IL)-1 receptor family that exists in at least 2 isoforms: a trans-membrane (ST2L) and a soluble circulating form (sST2). Although signaling between IL-33 and ST2L provides an important cardioprotective mechanism, sST2 acts as a decoy receptor of IL33, blocking cardioprotection and leading to myocardial fibrosis.
Data from one study showed that ST2 levels were significantly elevated in patients with AHF (P < .001) and also had prognostic value [Januzzi JL et al. J Am Coll Cardiol. 2007]. High ST2 levels also correlate with an increased risk of 1-year mortality [Rehman SU et al. J Am Coll Cardiol. 2008].
Dr Jaffe described ST2 as a “death marker” because it is a strong prognosticator for mortality, as further demonstrated by analysis of data from the GREAT network [Lassus J et al. Int J Cardiol. 2013]. He noted that higher levels of ST2 (> 35 ng/mL) may also identify patients at increased risk of 30-day hospital readmissions. Short-term changes in ST2 throughout time also provide prognostic value, with data showing that, during a 2-week period, increased elevation in sST2 levels increased the risk of mortality, whereas decreased levels reduced the risk of mortality (P < .001) [Bayes-Genis A et al. Rev Exp Cardiol. 2010]. Dr Jaffe also emphasized that unpublished data from his institution have shown that the use of gender-specific cutoff values improves risk stratification, compared with the clinically used single cutoff point of 35 ng/mL, for secondary end points such as rehospitalization.
One interesting observation is that ST2 concentration may be modified by treatments including β-blockers [Gaggin HK et al. J Am Coll Cardiol. 2013] and eplerenone [Weir AP et al. J Am Coll Cardiol. 2010]. The clinical implications of these observations will need to be clarified in future studies. Interesting data have also been reported, suggesting that ST2 and N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) combined may be associated with survivors of sudden death [Pascual-Figal DA et al. J Am Coll Cardiol. 2009]. Dr Jaffe emphasized, however, that there is no clear indication for routine clinical use of ST2 as yet.
Dr Peacock noted that assessment of cardiac troponin (cTn) levels should form part of early risk assessment because it provides important prognostic information for patients with AHF. He shared data showing that patients with high troponin levels required more cardiac procedures and longer hospitalization times, and were at high risk of in-hospital death [Peacock WF. N Engl J Med. 2008]
In another study that followed patients from hospital admission to discharge, high concentrations of troponin (> 23.25 ng/L list assay and 99th percentile) were associated with increased risk of readmission and mortality more than lower levels (< 23.25 ng/L; HR, 3.547; P = .003) [Xue Y et al. Eur J Heart Fail. 2011]. Evaluation of serial troponin levels in these patients also showed that progressively increasing levels in the first 3 measurements after admission comprised a poor prognostic indicator, predicting an increased risk of hospital readmission or death within 90 days, compared with patients whose troponin levels did not continue to increase.
Dr Peacock added that low levels of troponin may indicate subclinical chronic myocardial injury, and subsequently an increased risk of pathological cardiac remodeling and HF [de Lemos JA, Grundy SM. J Am Coll Cardiol. 2012]. An Italian study showed that cTn levels increased in association with LV hypertrophy (P < .0001) [Masson S et al. J Intern Med. 2013]. In another study, patients with this structural abnormality had increased levels of troponin that were reduced by diuretic treatment. Individuals who responded fastest to diuresis were successfully compensated, demonstrating decreased troponin levels (P = .025) that also correlated with decreased levels of NT-proBNP (P = .007) [Ferreira JP et al. Cardiol Res Pract. 2014]. Phase 3 data from the RELAX-AHF trial [Metra M et al. J Am Coll Cardiol. 2013] showed that patients with AHF in early serelaxin treatment experienced a reduction in 180-day mortality compared with those taking placebo [HR, 0.62; 95% CI, 0.43 to 0.88; P = .0076].
Consequently, an increase in troponin levels does appear to identify a cohort of patients at elevated risk of death who are more likely to have structural cardiac abnormalities and be less responsive to diuretic therapy. Dr Peacock concluded that clinical studies remain ongoing to determine whether it may also be a phenotypic target for specific intravenous neurohormonal antagonist therapies.
Dyspnea is one clinically defined phenotype in AHF, and, according to Alan Maisel, MD, Veterans Affairs Medical Center, San Diego, California, USA, pneumonia occurs with HF in up to 15% of patients. He presented data showing that this significantly increases the risk of in-hospital mortality (HR, 1.60; 95% CI, 1.38 to 1.85; P < .001) [Fonarow GC et al. Arch Int Med. 2008].
He emphasized the need for rapid evaluation of patients suspected to have pneumonia, to initiate antibiotic therapy, but highlighted the difficulty of diagnosing pneumonia in individuals with preexisting lung disease or AHF. Procalcitonin (PCT) has emerged, however, as one potentially useful protein biomarker with clinical value in the diagnosis of severe bacterial infection and sepsis, evaluation of its severity, and prediction of its course. Its level is low in healthy individuals (< 0.5 ng/mL), increases markedly in the presence of a bacterial-induced systemic inflammatory response (sepsis is likely with levels > 2 ng/mL), and decreases with effective treatment of the infection. Dr Maisel discussed studies showing its value in identifying the need for antibiotic therapy for pneumonia, including one demonstrating that PCT levels could be used to tailor antibiotic treatment duration to patients' individual needs [Christ-Crain M et al. Am J Resp Crit Care Med. 2006].
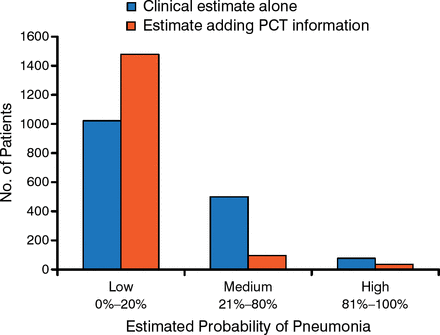
Dr Maisel advocated using serial assessment of biomarker levels in patients. He stressed, however, that biomarkers should not be used as stand-alone tests, but should be appropriately selected and combined to form panels to improve disease diagnosis. He also noted that PCT evaluation improves physicians' decision making about the presence of pneumonia in patients with HF (Figure 1) [Maisel A et al. Eur J Heart Fail. 2012].
Relationship Between Procalcitonin Assessment and Physician Decision Making
PCT, procalcitonin.
Adapted from Maisel A et al. Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnoea: results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur J Heart Fail. 2012;14:278–286. With permission from European Society of Cardiology.
According to Martin Möckel, MD, Charité University, Berlin, Germany, in the heart, adrenomedullin (ADM) is secreted predominantly by vascular cells. This protein provides an endocrine signal that is involved in pathway cross-talk among endothelial, smooth muscle, and cardiac muscle cells, resulting in vasodilatory, diuretic, and cardioprotective effects.
Prof Möckel noted that, due to its inherent instability, ADM is measured in plasma as the more stable prohormone, mid-region pro-adrenomedullin (MRpro-ADM). He added that MRpro-ADM levels are significantly elevated in patients with AHF and dyspnea, and strongly predict mortality, thereby defining a phenotype of patients at high risk of death. Prof Möckel also highlighted the value of combining biomarkers in certain scenarios, sharing data demonstrating increased diagnostic and prognostic value when MRpro-ADM and atrial natriuretic peptide are combined [Shah RV et al. Eur Heart J. 2010].
Although data from the BACH trial demonstrated value in the use of MRpro-ADM to guide disposition of patients with acute dyspnea [Moeckel M et al. Emerg Med J. 2013], Prof Möckel concluded that the therapeutic value of this biomarker also remains undetermined.
- © 2014 MD Conference Express®