Summary
In clinical practice, up to 25% of patients are statin intolerant as a result of symptoms and abnormalities in biomarkers. This article discusses results from the Study of Alirocumab (REGN727/SAR236553) in Patients With Primary Hypercholesterolemia and Moderate, High, or Very High Cardiovascular Risk, Who Are Intolerant to Statins [Odyssey Alternative; NCT01709513]. These trials compared statin intolerant patients who were treated with alirocumab versus ezetimibe.
- ODYSSEY
- lipid disorders
- cardiology clinical trials
Treatment of patients who are statin intolerant with alirocumab resulted in a significantly greater reduction in low-density lipoprotein cholesterol (LDL-C) when compared with ezetimibe. Patrick M. Moriarty, MD, University of Kansas Medical Center, Kansas City, Kansas, USA, presented these results from the Study of Alirocumab (REGN727/SAR236553) in Patients With Primary Hypercholesterolemia and Moderate, High, or Very High Cardiovascular Risk, Who Are Intolerant to Statins [Odyssey Alternative; NCT01709513].
In clinical practice, up to 25% of patients are statin intolerant as a result of symptoms and abnormalities in biomarkers [Mancini GB et al. Can J Cardiol. 2013; Cohen JD et al. J Clin Lipidol. 2012; Bruckert E et al. Cardiovasc Drugs Ther. 2005]. However, evidence from well-designed randomized trials is lacking for alternative cholesterol-lowering agents [Guyton JR et al. J Clin Lipidol. 2014]. The purpose of the Odyssey Alternative trial was to evaluate the efficacy and safety of the monoclonal antibody alirocumab in patients intolerant of statins.
In the double-blind phase 3 trial, 314 patients with statin intolerance were randomly assigned to receive alirocumab, ezetimibe, or atorvastatin for 24 weeks, followed by 2 years of open-label alirocumab treatment. Statin intolerance was defined as intolerance caused by muscle-related symptoms to a minimum of 2 statin drugs, including 1 at the lowest recommended dosage. All patients received placebo for 4 weeks before randomization. This was 1 of 3 periods of validation for these patients to determine their true intolerance to statins. During this period, 13% of patients dropped out, a majority because of muscle complaints. The patients who finished that section then were randomized into the blinded 24-week therapy section. These patients were blinded to alirocumab, ezetimibe, or atorvastatin for further validation of their intolerance to stains. At week 12, the dose of the study drug was increased if the LDL-C was ≥ 70 or ≥ 100 mg/dL, according to cardiovascular risk. The primary end point was percentage change in LDL-C from baseline in the alirocumab and ezetimibe arms.
At baseline, the mean age among all 3 cohorts was 63 years; slightly more than half were men; mean body mass index ranged from 28 to 30 kg/m2; and 7% of patients were current smokers. Hypertension was present in 62% of the patients, type 2 DM in 24%, and chronic heart disease in 46%. The mean LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglycerides were 191, 50, and 154 mg/dL, respectively.
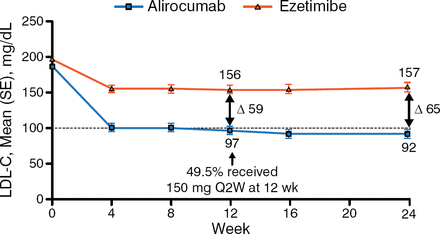
In the intention-to-treat population, patients who received alirocumab experienced a significantly greater decrease in LDL-C from baseline at week 24 as compared with patients who received ezetimibe (−45% vs −14.6%; P < .0001). The decrease in LDL-C occurred within 4 weeks of treatment and remained steady over the study period (Figure 1). In addition, 42% of patients achieved their LDL-C goal by week 24, compared with 4% in the ezetimibe arm (P <.0001). Other lipids—including non-HDL-C, apolipoprotein B, and lipoprotein (a)—demonstrated a greater reduction from baseline in the alirocumab arm versus the ezetimibe arm.
Effect of Alirocumab and Ezetimibe on Low-Density Lipoprotein Cholesterol Over 24 Weeks
LDL-C, low-density lipoprotein cholesterol; Q2W, every other week.
Reproduced with permission from PM Moriarty, MD.
In the safety analysis, a similar number of patients experienced treatment-emergent adverse events (TEAEs), with 18%, 25%, and 25% discontinuing alirocumab, ezetimibe, and atorvastatin, respectively, because of TEAEs. The number of skeletal muscle TEAEs was significantly different between the alirocumab and atorvastatin arms, (P < .042) but total discontinuation occurred in 19% of patients, with a similar number occurring in all 3 arms of the study. Common adverse events included myalgia, nasopharyngitis, arthralgia, upper respiratory tract infection, headache, fatigue, muscle spasms, back pain, paresthesia, vomiting, and muscular weakness. The 14-week interim analysis of the open label alirocumab period has indicated that <3% of the patients have dropped out because of TEAEs.
Dr Moriarty concluded that the data from the Odyssey Alternative trial indicate that alirocumab had greater efficacy than ezetimibe at week 24 for the reduction of LDL-C, with fewer TEAEs, including fewer skeletal muscle events. Additionally, the unpredictable nature of patients' intolerance to alirocumab, ezetimibe, and atorvastatin in the 4-week placebo period and 24-week blinded therapy period demonstrates the complexity of diagnosing and treating patients with statin intolerance.
- © 2014 MD Conference Express®