Summary
The results of the multicenter TAXUS Liberté Post Approval Study [TL-PAS] are discussed in this article. Patients randomized in this prospective open-label study were also participants in the Dual Antiplatelet Therapy Study. While the TL-PAS was not powered to show a difference in the end point analyses, the results from this specific trial were presented.
- interventional techniques & devices
- thrombotic disorders
- cardiology clinical trials
The results of the multicenter TAXUS Liberté Post Approval Study [TL-PAS; Garratt KN et al. Circulation. 2014] were presented by Kirk Garratt, MD, Lenox Hill Hospital, New York, New York, USA. Patients randomized in this prospective open-label study were also participants in the Dual Antiplatelet Therapy Study [Mauri L et al. New Eng J Med. 2014]. While the TL-PAS was not powered to show a difference in the end point analyses, the results from this specific trial were presented.
Eligible patients had a TAXUS Liberté paclitaxel-eluting stent placed and then took open-label aspirin plus prasugrel for 12 months after the index procedure [Garratt KN et al. Circulation. 2014]. At the end of 12 months, patients were randomized to blinded prasugrel (30-month group) or placebo (12-month group) plus open-label aspirin for another 18 months. Thirty months after the index event, blinded study medication was stopped, and the patients took aspirin for ≥ 3 months.
The co-primary efficacy end points were the occurrence of major adverse cardiac and cerebrovascular events (defined as the composite of death, myocardial infarction [MI], or stroke) and stent thrombosis (ST) occurring between 12 and 30 months after the index procedure. The primary safety end point was major bleeding occurring between 12 and 30 months after the index procedure (defined as moderate or severe by GUSTO criteria).
Of the 2191 randomized patients, about 75% were men, and the mean age was approximately 59 years; >96% were aged < 75 years and weighed > 60 kg.
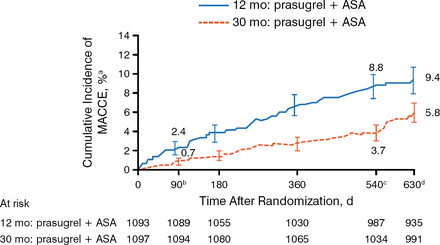
At 540 days after randomization, patients in the 30-month prasugrel group had a significantly lower rate in the co-primary composite end point of death, MI, or stroke when compared with the 12-month group (Figure 1). This difference was also significant at the 90-day postrandomization time point (P = .002). The co-primary end point of ST was also lower in patients taking prasugrel for 30 months versus the 12-month group.
Co—Primary End Point: Major Adverse Cardiac and Cerebrovascular Events at 540 Days
ASA, acetylsalicylic acid (aspirin); MACCE, major adverse cardiac and cerebrovascular event.
a±1.5 SE.
bHR, 0.303; 95% CI, 0.137 to 0.670; P =.002.
cHR, 0.407; 95% CI, 0.281 to 0.589; P <.001.
dHR, 0.591; 95% CI, 0.431 to 0.811; P <.001.
Adapted from Garratt KN et al. Prasugrel plus aspirin beyond 12 months is associated with improved outcomes after Taxus Liberté paclitaxel-eluting coronary stent placement. Circulation. 2015. E-pub ahead of print. DOI: 10.1161/CIRCULATIONAHA.114.013570. Accessed December 10, 2014. With permission from American Heart Association, Inc.
There were no significant differences in the rates of stroke or death, but patients in the 30-month group had significantly fewer MIs (P <.001). An increase in major bleeds was observed with prolonged prasugrel therapy, but the increase was not statistically significant, and the rate of severe bleeds was not higher in the 30-month treatment group. An important finding was that stopping prasugrel appeared to result in a loss of protection, as an increase in ischemic events was seen within 90 days of discontinuation in both arms. Key effectiveness and safety study results are presented in Table 1.
Key TL-PAS Results at 540 Days Postrandomization
Dr Garratt noted that the trial has several limitations. Patients with a history of prior cerebrovascular or active bleeding events were excluded, and those patients who were randomized had demonstrated tolerance to prasugrel for 12 months. In addition, elderly patients or those with a lower body mass may have been under-represented. However, these data demonstrate that long-term prasugrel reduces ischemic events while increasing the risk of bleeding. Furthermore, these data provide additional evidence that cessation of antiplatelet agents increases the short-term risk of ischemic events during the period immediately following cessation of therapy.
- © 2014 MD Conference Express®