Summary
The current standard classification criteria for systemic sclerosis (SSc; scleroderma) were published in 1980 based on studies beginning in 1975. Since that time our knowledge concerning SSc has improved, particularly with respect to antibodies and nailfold changes, and the need for a new classification system has become evident. At the American College of Rheumatology (ACR) 2014 Meeting, representatives of a joint committee of the ACR and the European League Against Rheumatism (EULAR) presented a proposed new classification criteria for SSc [van den Hoogen F et al. Arthritis Rheum 2013].
- Rheumatology Guidelines
- Rheumatological Autoimmune Disorders
- Rheumatology
- Rheumatology Guidelines
- Rheumatological Autoimmune Disorders
- Rheumatology
The current standard classification criteria for systemic sclerosis (SSc; scleroderma) were published in 1980 based on studies beginning in 1975. Since that time our knowledge concerning SSc has improved, particularly with respect to antibodies and nailfold changes, and the need for a new classification system has become evident. At this year's meeting of the American College of Rheumatology (ACR), representatives of a joint committee of the ACR and the European League Against Rheumatism (EULAR) presented a proposed new classification criteria for SSc, which performs better than the 1980 ACR criteria and should allow for more patients to be correctly classified [van den Hoogen F et al. Arthritis Rheum 2013].
The analysis leading to the development of these criteria included half the patients with early SSc (which is different than the original study), and has better sensitivity and specificity, and is relatively simple to apply to individual subjects. Both organizations have endorsed the new criteria. Frank H.J. van den Hoogen, MD, PhD, Sint Maartenskliniek and Radboud University Medical Centre, Nijmegen, The Netherlands, discussed the criteria in terms of need and how to use the new classification scheme.
The object of the current project was to develop a set of criteria that would enable identification of individuals with SSc for inclusion in clinical studies and be more sensitive and specific than the 1980 criteria. Specific aims were that the criteria should include a broad spectrum of SSc (ie, patients with early- and late-stage disease) and include vascular, immunologic, and fibrotic manifestations. The ideal criteria should be feasible for use in daily clinical practice and in accordance with the criteria used for diagnosis. Finally, the criteria are intended to be used by rheumatologists, other specialists, researchers, national and international drug agencies, pharmaceutical companies, and/or any others involved in studies of SSc.
The task force used a combination of expert opinion and data-driven methodology to develop and validate the criteria. Data from two Delphi exercises initially identified 168 potential features of SSc (items) for classification. A second Delphi exercise was used to reduce these to 23 for further analysis [Fransen J et al. Arthritis Care Res 2012]. Ranking and choice analysis reduced the items to 14, then eight with skin thickening of the fingers, finger-tip lesions, scleroderma-related autoantibodies, and Raynaud's phenomenon listed as some of the top criteria. Any patient with scleroderma skin changes of the fingers and proximal to themetacarpophalangeal joints is already classified as SSc. The items and weight of each item selected for the use in final classification criteria are shown in Table 1. “Either/or” choices of puffy or sclerodactyly of the fingers for skin thickening of the fingers and digital tip ulcer or fingertip pitting scar for fingertip lesions were included as subitems. A total score ≥9 is needed to classify a patient as having definite SSc. The criteria are applicable to any patient considered for inclusion in an SSc study, but not to patients having an SSc-like disorder better explained by different manifestations or patients with skin thickening sparing the fingers.
ACR-EULAR SSc Classification Criteria and Weights
The sensitivity and specificity of the 2013 criteria was improved when compared with the prior classification system (Table 2). Prof. van den Hoogen encouraged the validation of the 2013 criteria in other cohorts of patients.
Sindhu R. Johnson, MD, PhD, University of Toronto, Toronto, Ontario, Canada, provided additional details concerning the methodology used to develop the new criteria. She discussed the guiding principles underlying the methodology, identified the methodologic framework, and highlighted the main findings of each phase.
Sensitivity and Specificity in SSc Cases and Controls
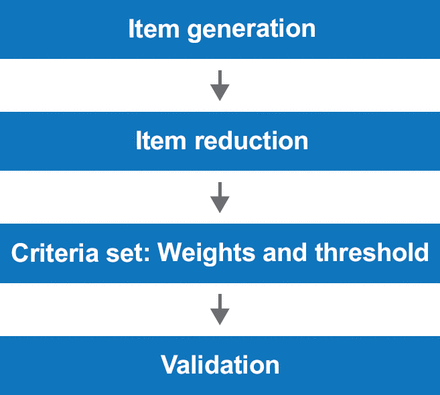
An important objective in the methodology was to avoid “circular reasoning”—a situation in which experts develop criteria based on their own patient population and then validate the criteria in that same population. To achieve this, the committee followed the 4-step process shown in Figure 1. Item generation was performed using data from two different Delphi exercises in which experts from the Scleroderma Clinical Trials Consortium (SCTC) and the EULAR Scleroderma Trials and Research (EUSTAR) network were asked similar but slightly different questions (one set focusing on diagnosis in daily practice, the other focusing on early diagnosis). This process generated the previously discussed 168 candidate criteria. Further reduction was accomplished through a combined Delphi exercise in which 106 SCTC and EUSTAR experts were asked to rank the 168 items on a scale of 1 to 9. Items with a median score of <4 were eliminated leaving 102 criteria for further analysis. These were reduced to 23 during a face-to-face meeting of 16 experts [Fransen J et al. Arthritis Care Res 2012]. The 23 candidate items were validated and shown to have good face, discriminant, and construct validity. Empirical and expert ranking were correlated (Spearman's rho=0.53; p=0.01) [Johnson SR et al. Arthritis Care Res 2012].
Methodologic Framework
Reproduced with permission from SR Johnson, MD, PhD.
The next phase required aggregating the criteria, ascertaining the weights, and defining the threshold. Instrument development involved design and sensibility testing (clarity of instructions, clarity of the form and response options, and ease of navigation of the form) used for validation of the system. The Potentially All Pairwise Rankings of All Hypothetically-Possible Patients (PAPRIKA) method was used for ranking and multi-criteria decision analysis. Criteria with low weights were eliminated. The strengths of this approach include the rigor of the methodology (bias reduction and large number of investigators and subjects) and diversity (consensus methods, measurement science, and multi-criteria decision analysis).
Janet E. Pope, MD, MPH, Western University and St. Joseph's Health Care, London, Ontario, Canada, reviewed the operational use of the new classification criteria and discussed some of its limitations.
The new criteria are an improvement over the 1980 version in that they have good face, discriminant, and construct validity, and their sensitivity and specificity are better. The new criteria outperform the 1980 criteria for patients in the Canadian Scleroderma Research Group Cohort [Alhajeri H et al. ACR 2013 (abstr 1810)] and in the Norwegian Systemic Connective Tissue and Vasculitis Registry [Hoffman-Vold A et al. ACR 2013 (abstr 690)].
Using the new criteria, patients can be classified with more than one disease and the system can identify more patients who would not have been classified by previous criteria. The new criteria are not ideal in that they are still not the same as diagnostic criteria, meaning that some patients diagnosed with SSc may not meet the classification criteria. For example, some patients may be excluded in the absence of sclerodactyly if skin involvement is elsewhere.
Prof. Pope cautioned about the necessity to mark the highest score, when more than one item in a single class is present. She also noted that higher scores do not necessarily mean more severe disease. The new criteria should not be used to assign a diagnosis of SSc if there is a better explanation for the disease, such as generalized morphea. Patients with previous evidence of dilated nailfold capillaries, which is no longer present, can be scored as having this item. So, an item can be scored if it ever occurred and the rheumatologist is certain of that, even if not present now. Patients are allowed to have an overlap (ie, SSc and another connective tissue disease or rheumatoid arthritis).
The new classification system has evolved over the years with the help of advancing technology such as capillaroscopy, autoantibodies, and new methodologies. One draw back is that not all centers have access to RNA polymerase III Abs to perform antibody tests.
- © 2013 MD Conference Express®