Summary
According to the results of the multivitamin arm of the Trial to Assess Chelation Therapy [TACT; NCT00044213], high-dose vitamins alone do not improve outcomes for postmyocardial infarction patients.
- Myocardial Infarction
- Cardiology Clinical Trials
- Myocardial Infarction
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
According to the results of the multivitamin arm of the Trial to Assess Chelation Therapy [TACT; NCT00044213], presented by Gervasio A. Lamas, MD, Mount Sinai Medical Center, Miami Beach, Florida, USA, high-dose vitamins alone do not improve outcomes for postmyocardial infarction (MI) patients.
TACT was a randomized, double-blind, placebo-controlled, 2×2 factorial clinical trial testing the clinical impact of 40 infusions of a multi-component Na2EDTA-chelation solution and/or high-dose multivitamin and mineral supplement in patients with acute MI. The TACT trial had 4 treatment arms: placebo infusions/placebo vitamins; placebo infusions/high-dose vitamins; EDTA chelation/placebo vitamins; and EDTA chelation/high-dose vitamins. It involved 1708 post-MI patients at least 50 years of age. Approximately a third of the patient population was diabetic. The trial was designed to have >85% power to detect a 25% relative reduction in the primary endpoint for each treatment factor [Lamas GA et al. Am Heart J 2012]. The primary endpoint was a composite of time to first occurrence of death, MI, stroke, coronary revascularization, or hospitalization for angina.
The TACT multivitamin primary endpoint results were not significant (Table 1). Use of statins at baseline was the only predefined subgroup that interacted with the oral vitamin therapy, suggesting that patients who self-selected to not take statins (27% of the total) might have a better response to active oral vitamins (p for interaction=0.01). Dr. Lamas cautioned that these subgroup results are hypothesis generating only.
Components of the Primary Endpoint
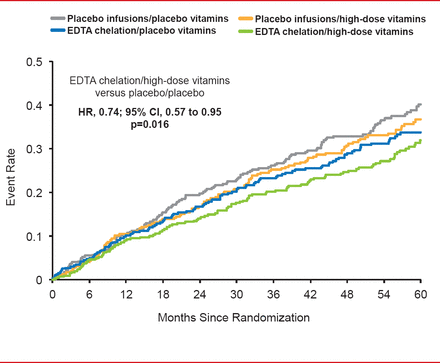
When the 4 treatment groups were compared, a statistically significant difference of clinically interesting magnitude emerged between placebo infusion/placebo vitamins and active/active therapies (HR, 0.74; 95% CI, 0.57 to 0.95; p=0.016; Figure 1).
TACT Primary Endpoint in the Study's 4 Treatment Arms
EDTA=ethylenediaminetetraacetic acid.
Dr. Lamas noted that despite the evident benefit, the findings are insufficient to recommend the routine use of chelation therapy and high-dose vitamins in post-MI patients. He also reported that the study results do not support the use of high-dose vitamin and mineral therapy as an adjunct to optimal evidence-based medical therapy in patients with prior MI.
Limitations of the trial included constrained power to assess individual components of the composite endpoint; a high vitamin noncompliance rate; many subjects were lost to follow-up and withdrew consent; and potential confounding by oral vitamins and minerals. The hypothesis of proposed benefit of high-dose vitamin therapy was also unclear, which limited interpretation.
Earlier randomized, controlled trials of specific supplements failed to demonstrate a consistent or significant effect of any single or combination of vitamins on incidence of death from cardiovascular disease [Morris CD, Carson S. Ann Intern Med 2003]. Dr. Lamas concluded that the results of the study do not support the use of high-dose vitamin and mineral therapy as an adjunct to optimal evidence-based medical therapy in patients with acute MI but should serve as an impetus for further research.
- © 2013 MD Conference Express®