Summary
The absence of Phase 3 trials showing a mortality benefit from pharmacological treatment of acute lung injury (ALI) has led to a shift toward identifying patients who are at risk for lung injury and employing risk modification and treatment to prevent progression to respiratory failure or the need for mechanical ventilation. Such an approach includes the use of novel pharmaceutical agents in addition to those previously used unsuccessfully to treat established acute respiratory distress syndrome. This strategy also includes identifying and treating at-risk patients in the emergency department or even perioperatively and is not limited to targeting patients in the intensive care unit.
- Acute Lung Injury & Respiratory Failure
- Featured Meeting - Specialty page
- Pulmonary Genomics
- Pulmonary & Critical Care
- Acute Lung Injury & Respiratory Failure
- Featured Meeting - Specialty page
- Pulmonary Genomics
The absence of Phase 3 trials showing a mortality benefit from pharmacological treatment of acute lung injury (ALI) has led to a shift toward identifying patients who are at risk for lung injury and employing risk modification and treatment to prevent progression to respiratory failure or the need for mechanical ventilation. Such an approach includes the use of novel pharmaceutical agents in addition to those previously used unsuccessfully to treat established acute respiratory distress syndrome (ARDS). This strategy also includes identifying and treating at-risk patients in the emergency department or even perioperatively and is not limited to targeting patients in the intensive care unit.
The lung injury prediction score (LIPS), based on predisposing conditions and risk modifiers, is a well-validated scoring mechanism used to identify patients at risk for ALI [Gajic O et al. Am J Respir Crit Care Med 2010]. However, the LIPS requires inclusion of multiple risk modifiers which may be cumbersome to calculate in clinical practice and with a positive predictive value of only 18%, identifies a relatively low at-risk cohort.
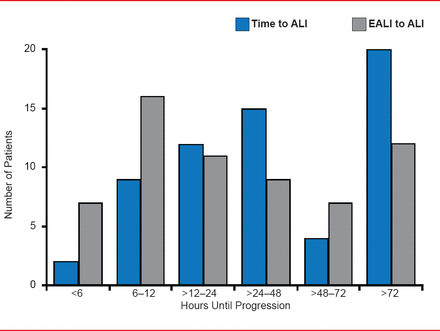
Joseph Levitt, MD, MS, Stanford University Medical Center, Stanford, California, USA, described another scoring system developed at Stanford for early ALI (EALI). The system is based on only three predictive variables: supplemental oxygen requirements (number of liters of oxygen required to keep saturation level above 90%), respiratory rate, and immune suppression. An oxygen requirement of either >2 to 6 liters/minute or >6 liters/minute, a respiratory rate of ≥30 breaths/minute, and evidence of immune suppression are significant (p≤0.02) independent predictors of progression to ALI. EALI employs a 0 to 4 point scoring system (one point for each independent risk factor) and has a slightly better discrimination score (0.87) than either LIPS (0.83) or the Acute Physiology and Chronic Health Evaluation II (APACHE II; 0.67). The time from the initial chest x-ray meeting the criteria for potential lung injury to the development of ALI (blue bars) and the time from first meeting EALI >2 score criteria to ALI development (orange bars) is shown in Figure 1. Median time from EALI score ≥2 to ALI was 20 hours, which provides a clinically relevant time frame for successful intervention. However, EALI may not identify some high-risk patients such as postoperative patients without evidence of lung injury prior to surgery or patients with rapid progression and needs external validation.
ARDS is a postoperative complication associated with poor survival. Prevention of postoperative ARDS is achievable if patients at risk can be identified preoperatively. Daryl Kor, MD, Mayo Clinic, Rochester, Minnesota, USA, discussed the baseline and intraoperative risk factors his team developed for identifying at-risk patients [Kor DJ et al. Anesthesiology 2011].
Time of Progression From EALI to ALI
ALI=acute lung injury; EALI=early acute lung injury.
Reproduced from Levitt J et al. Early Acute Lung Injury: Criteria for Identifying Lung Injury Prior to the Need for Positive Pressure Ventilation. Critical Care Medicine 2013;41(8). With permission the Society of Critical Care Medicine and Lippincott Williams & Wilkins.
The surgical lung injury prediction score can distinguish patients who are likely to develop early postoperative ARDS from those who will not (AUC, 0.82; 95% CI, 0.78 to 0.86). Significant preoperative predictive factors include high-risk cardiac, aortic vascular, and thoracic surgery (all p<0.01). Baseline comorbidities/risk modifiers include chronic obstructive pulmonary disease, diabetes mellitus, gastroesophageal reflux disease, and alcohol abuse. Additional research suggests active infectious/inflammatory processes, preoperative chemoradiation and tobacco abuse may also portend risk for postoperative ARDS.
Additional intraoperative predictive factors including the complexity/duration of the surgical procedure, elevated airway pressures, high tidal volume, shock, liberal fluid resuscitation strategies, and liberal transfusion strategies continue to be explored. For instance, among patients without ARDS, protective ventilation with lower tidal volumes is associated with better clinical outcomes and a lower ARDS incidence rate.
The incidence of postoperative ARDS is decreasing. Two reasons suggested for the decline are reductions in the number of transfusions administered during a procedure and a change in transfusion donor policy. Specifically, evidence suggests a reduced rate of ARDS following the change to male-only fresh frozen plasma donor policies in 2003 (36% before vs 21% after; p=0.04) [Chapman CE et al. Transfusion 2009]. Further evaluation of intraoperative and postoperative risk predictors and the identification of intermediate markers for ARDS are needed.
New biomarkers that would help predict and diagnose patients at highest risk for ARDS, reduce heterogeneity, and serve as surrogate outcome measures for clinical trials are needed, stated Carolyn Calfee, MD, MAS, University of California, San Francisco, San Francisco, California, USA.
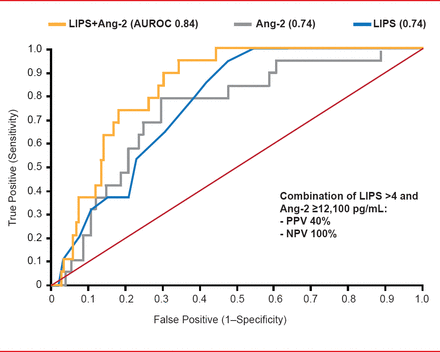
Early intervention often confers outcomes that are more successful. The Early Assessment of Renal and Lung Injury [EARLI] cohort study identified high levels of plasma angiopoietin-2 (Ang-2) and interleukin-8 (IL-8) as early predictors of patients at high risk for ALI [Agrawal A et al. Am J Respir Crit Care Med 2013]. The combination of LIPS (score >4) and Ang-2 proved superior to either alone (AUROC 84; p=0.05; Figure 2).
Combining clinical and biologic markers for diagnosis and prediction of clinical outcomes has been of major value in many clinical disorders. A recent study suggested that a combination of two biological markers (SPD and IL), age, and severity of illness (APACHE score) might be useful for predicting ALI/ARDS mortality [Ware LB et al. Chest 2010].
The declining mortality trend in ALI signals the need for surrogate endpoints that could allow for more efficient clinical trial design. In one study, infusion of intravenous salbutamol accelerated the resolution of alveolar edema (as measured by extravascular lung water [EVLW]) in adult patients with ALI or ARDS, suggesting that EVLW might be a good surrogate marker [Perkins GD et al. Am J Respir Crit Care Med 2006]. Further research, however, showed that early treatment with salbutamol increased 28-day morality in ARDS patients [Gao Smith F et al. Lancet 2012]. Thus, the search for surrogate biomarkers remains challenging.
The median time to ALI/ARDS is 2 days post admission and there is a critical point at which intervention can reduce the incidence of ARDS if the most susceptible patients can be identified at the right time [Kor DJ. BMJ Open 2012]. However, variations in patient management may be high as patients transition from the emergency department to the ward to the intensive care unit.
Such variation can lead to experimental noise and heterogeneity that can make it more difficult to detect an effect in clinical trials.
If adhered to, standardized processes of care can decrease experimental noise and reduce variability in the development of ARDS. Unneccessary transfusions, high tidal volume, aspiration, and delayed antibiotic treatment have been associated with increased development of ARDS. Early goal directed resuscitation decreases the likelihood of acute respiratory failure requiring ventilation, but compliance with early goal directed therapy also varies from patient to patient, clinician to clinician, and between institutions and organizational levels.
LIPS Plus Ang-2 Superior to Either Alone for ALI Prediction
LIPS=lung injury prediction score; Ang-2=angiopoietin-2; AUROC=area under the receiver operating characteristic; NPV=negative predictive value; PPV=positive predictive value.
Reprinted with permission of the American Thoracic Society. Perkins GD et al. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006 Feb 1;173(3):281–7. Official journal of the American Thoracic Society.
A checklist that is adaptable to different institutions, multiple teams in different areas of the hospital, and different and changing conditions of the patient is one approach that can be used to standardize the processes of care that can increase the risk of ARDS. Michelle Gong, MD, MS, Albert Einstein College of Medicine, Bronx, New York, USA, described an interactive electronic checklist called Checklist for Lung Injury Prevention (CLIP) used in the LIPS-A study. The CLIP can improve compliance with recommendations such as low tidal volume in vented at-risk patients early in their critical illness even when they are still down in the emergency department (p=0.07). It may not be possible to achieve 100% compliance for many standardized practices and protocols in critical care trials.
- © 2013 MD Conference Express®