Summary
This article discusses the evidence from clinical trials addressing the reduction of cardiovascular (CV) risk with antihyperglycemic drugs. Specific trials include the UKPDS, ACCORD, RECORD, NAVIGATOR, and ORIGIN trials, as well as a number of ongoing clinical trials examining the CV safety of dipeptidyl peptidase-4 inhibitors.
- Cardiology Clinical Trials
- Hypertensive Disease
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
- Hypertensive Disease
The evidence from clinical trials addressing the reduction of cardiovascular (CV) risk with antihyperglycemic drugs was discussed by Peter M. Nilsson, MD, PhD, Lund University, Malmö, Sweden.
Metformin became first-line therapy for type 2 diabetes mellitus (T2DM) after the findings of UKPDS34, which showed a 39% relative reduction in myocardial infarction (MI) in overweight T2DM patients (n=342) treated with the drug versus those treated with conventional treatment (RR, 0.61; 95% CI, 0.41 to 0.89; p=0.01) [UK Prospective Diabetes Study Group. Lancet 1998]. A meta-analysis of 12 randomized trials, including data on metformin, showed that it was beneficial for CV events versus placebo or no treatment in patients with T2DM (OR, 0.79; 95% CI, 0.64 to 0.98; p=0.031) but similar to active comparators [Lamanna C et al. Diabetes Obes Metab 2011]. An overall comparison of metformin versus placebo, no treatment, or active comparator also found no significant difference between the groups. Yet, according to Prof. Nilsson, metformin should remain a first-line drug.
In the ACCORD trial, intensive therapy was associated with a positive but nonsignificant benefit on the primary composite outcome of nonfatal MI, nonfatal stroke, or CV death in T2DM patients with CV disease (CVD) or CV risk factors when compared with standard therapy (HR, 0.90; 95% CI, 0.78 to 1.04; p=0.16) [ACCORD Study Group. N Engl J Med 2008]. An increased risk in all-cause mortality was found with intensive therapy versus standard therapy (HR, 1.22; 95% CI, 1.01 to 1.46; p=0.04). This surprising finding spurred several meta-analyses to evaluate the potential effects of antihypertensive drugs on CV endpoints in T2DM, said Prof. Nilsson.
A meta-analysis of 5 studies, including UKPDS and ACCORD, showed a 17% reduction in the risk of nonfatal MI (OR, 0.83; 95% CI, 0.75 to 0.93) and a 15% reduction in the risk of coronary heart disease (OR, 0.85; 95% CI, 0.77 to 0.93) with intensive glucose lowering versus standard treatment with an antihypertensive agent [Ray KK et al. Lancet 2009]. The 33,040 T2DM patients in this meta-analysis were followed for a mean of 4.95 years. There were no significant differences between the two groups in the risk of stroke (OR, 0.93; 95% CI, 0.81 to 1.06) or all-cause mortality (OR, 1.02; 95% CI, 0.87 to 1.19).
The RECORD trial showed no difference in the outcome of CV hospitalization or CV death with rosiglitazone during a mean follow-up of 5.5 years compared with a combination of metformin and sulfonylurea in T2DM patients who were already on metformin or sulfonylurea monotherapy (HR, 0.99; 95% CI, 0.85 to 1.16; p=0.93) [Home PD et al. Lancet 2009]. The annual event rate was about 2.8% for each drug. Rosiglitazone was removed from the market because of these results. In June 2013, based on the required readjudication of CV events in RECORD, the FDA panel eased the restrictions on its use [American Heart Association. Press Release. http://newsroom.heart.org/news/fda-panel-recommends-easing-avandia-restrictions. Published June 06, 2013].
Nateglinide, a new insulin secretor drug, did not reduce CV outcomes compared with placebo in the NAVIGATOR study [NAVIGATOR Study Group. N Engl J Med 2010]. In patients with T2DM or impaired fasting glucose, and CVD or CV risk factors, the occurrence of the outcomes was similar in both nateglinide and placebo groups for the extended CV endpoint (15.2% vs 14.2%; HR, 0.93; 95% CI, 0.83 to 1.03; p=0.16) as well as the core CV endpoint (7.9% vs 8.3%; HR, 0.94; 95% CI, 0.82 to 1.09; p=0.43) over a median follow-up of 6.3 and 6.4 years, respectively. Prof. Nilsson stated that it is a challenge to obtain benefit with add-on therapy with new drugs because T2DM patients are well managed with current treatments.
In the ORIGIN trial, 12,537 patients aged ≥50 years with dysglycemia and high CV risk were randomized to insulin glargine or standard care and followed up to a median of 6.2 years [ORIGIN Trial Investigators. N Engl J Med 2012]. Both coprimary endpoints were neutral. The first coprimary outcome was CV death, nonfatal MI, or nonfatal stroke (adjusted HR, 1.02; 95% CI, 0.94 to 1.11; p=0.63). The second coprimary endpoint was CV death, nonfatal MI, nonfatal stroke, revascularization, or hospitalization for heart failure (adjusted HR, 1.04; 95% CI, 0.97 to 1.11; p=0.27). The reduction in median HbA1c favored glargine (6.2%) over standard treatment (6.5%) at 7 years.
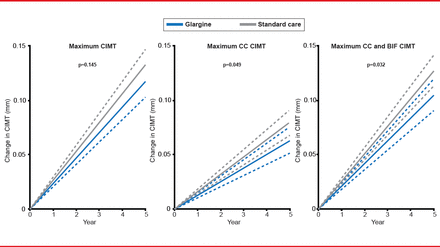
A post hoc analysis of ORIGIN [ORIGIN-GRACE] showed slower progression of carotid intima-media thickness as measured by ultrasound with insulin glargine compared with standard treatment (Figure 1) [Lonn EM et al. Diabetes Care 2013]. Although this has a positive effect on target organ damage, it is not known whether this finding would translate to a reduction in CV events, said Prof. Nilsson.
Progression of Carotid Intima Media Thickness
BIF=bifurcation; CC=common carotid; CIMT=carotid intima-media thickness.
Reproduced from Lonn EM et al. Effect of Insulin Glargine and n-3FA on Carotid Intima-Media Thickness in People With Dysglycemia at High Risk for Cardiovascular Events: The Glucose Reduction and Atherosclerosis Continuing Evaluation Study (ORIGIN-GRACE). Diabetes Care 2013. With permission from the American Diabetes Association.
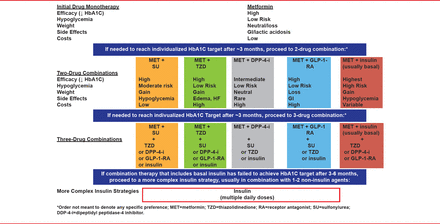
The current treatment algorithm for T2DM from the American Diabetes Association and the European Association for the Study of Diabetes is shown in Figure 2 [Inzucchi SE et al. Diabetes Care 2012].
The ADA/EASD Treatment Algorithm for T2DM
ADA=American Diabetes Association; EASD=European Association for the Study of Diabetes.
Reproduced from Inzucchi SE et al. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered Approach: Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35:1364–1379. With permission from the American Diabetes Association.
Regarding second-line drug choices, there are no trial data to show that one drug class is superior to another, said Prof. Nilsson. He noted that some of the antihyperglycemic agents that have a positive effect on lipids are insulin, metformin, glitazones for dyslipidemia, and incretins.
There are a number of ongoing clinical trials examining the CV safety of dipeptidyl peptidase-4 inhibitors (linagliptin [CAROLINA; NCT01243424], sitagliptin [TECOS; NCT00790205], saxagliptin [SAVOR-TIMI-53; NCT01107886], and alogliptin [EXAMINE; NCT00968708]), and some with insulin endocrine analogues. In particular, the results of the randomized, double-blind, SAVOR-TIMI-53 study with saxagliptin in >16,000 T2DM patients are anticipated soon [Scirica BM et al. Am Heart Journal 2011]. This is the first real test of the new principal of influencing the endocrine system, said Prof. Nilsson.
The new and more effective antihyperglycemic drugs exploring new mechanisms could have the potential to be more effective for CV prevention.
- © 2013 MD Conference Express®