Summary
Four to six cycles of platinum-based doublet chemotherapy remains the standard treatment protocol for most patients with advanced non-small cell lung cancer (NSCLC). Longer treatment does not appear to increase overall or progression-free survival and may lead to toxicity issues and reduced quality of life. This article discusses the current state of knowledge concerning treatment for NSCLC patients without a targetable (driver) mutation, the impact of oncogenic drivers on treatment of NSCLC, as well as the use of maintenance therapy for patients with cancer as a way to delay disease progression and improve survival.
- Oncology Genomics
- Respiratory Cancers
- Cancer
- Oncology Genomics
- Oncology
- Respiratory Cancers
- Cancer
Four to six cycles of platinum-based doublet chemotherapy remains the standard treatment protocol for most patients with advanced non-small cell lung cancer (NSCLC). Longer treatment does not appear to increase overall (OS) or progression-free survival (PFS) and may lead to toxicity issues and reduced quality of life (QoL). Frances A. Shepherd, MD, FRCPC, University of Toronto, Princess Margaret Hospital, Toronto, Ontario, Canada, discussed the current state of knowledge concerning treatment for NSCLC patients without a targetable (driver) mutation.
After an initial diagnosis of NSCLC some of the issues in the selection of treatment of first-line therapy include the impact of histology on the selection of a regimen, treatment of the elderly, addition of a targeted agent, and whether to use chemotherapy or a targeted agent. Even with full immunohistochemistry testing, subtyping may not be possible in 10% to 15% of cases, but testing can be used to identify patients with squamous histology in whom bevacizumab is associated with increased toxicity (hemoptysis) and pemetrexed has inferior results [Scagliotti GV et al. J Clin Oncol 2008].
Traditionally, older patients (>70 years) have been treated with monotherapy, but recent studies have shown that platinum-based doublet chemotherapy is associated with significantly (p<0.0001) improved survival benefits (10.3 months) compared with vinorelbine or gemcitabine monotherapy (6.2 months) [Quoix E et al. Lancet 2011].
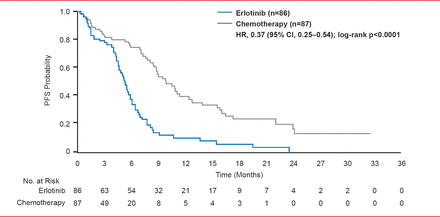
The addition of targeted agents, like bevacizumab and cetuximab, to first-line chemotherapy doublets can add survival benefits in selected (nonelderly fit patients) but comes with an increased risk of treatment-related deaths [Sandler AB et al. N Engl J Med 2006]. Routine initial tissue-based assessment of epidermal growth factor receptor (EGFR) mutations in NSCLC patients followed by treatment of mutation-positive patients with EGFR tyrosine kinase inhibitors (TKIs), such as erlotinib or gefitinib, improves PFS (p<0.0001) compared with chemotherapy (Figure 1) [Rosell R et al. Lancet Oncol 2012]. In patients with advanced pulmonary adenocarcinoma who are negative for the mutation, PFS is significantly longer (p<0.001) with chemotherapy versus gefitinib [Mok TS et al. N Engl J Med 2009].
PFS in Intention to Treat Population
Reproduced from Rosell R et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncology 2012;13(3):239–246. With permission from Elsevier.
Crizotinib, an anaplastic lymphoma kinase (ALK) inhibitor, has been approved for use in NSCLC patients with ALK mutations. In this patient group, it significantly improves (p<0.0001) PFS compared with chemotherapy [Shaw A et al. N Engl J Med 2013]
A number of molecular tests have been put forward as prognosticators to select or exclude chemotherapy such as KRAS and ERCC1 in NSCLC patients, but the results of these tests remain inconclusive. Prof. Shepherd cautioned that to date “there is no molecular test to select or exclude chemotherapy for patients without a driver mutation.”
Paul A. Bunn, Jr., MD, University of Colorado School of Medicine, Denver, Colorado, USA, discussed the impact of oncogenic drivers on treatment of NSCLC.
In 2004, two groups identified specific mutations in the EGFR gene, which correlate with clinical responsiveness to the TKIs gefitinb and erlotinib. These mutations lead to increased growth factor signaling and confer susceptibility to the inhibitor. Screening for such mutations in lung cancers may identify patients who will have a response to gefitinib [Pao W et al. Proc Natl Acad Sci 2004; Lynch TJ et al. N Engl J Med 2004; Paez JG et al. Science 2004]. In the IPASS trial, gefitinib was shown to prolong PFS in patients with EGFR mutations but not in patients without EGFR mutations and to improve both QoL and symptoms [Thongprasert S et al. J Thorac Oncol 2011]. Several other trials have gone on to show that use of an oral TKI leads to superior response (but not OS) compared with platinum doublet chemotherapy as first-line treatment for EGFR mutant patients.
Both European and American oncology associations guidelines recommend that patients with advanced NSCLC be tested for active EGFR mutations before receiving first-line therapy and that the choice of an EGFR TKI or chemotherapy should be based on the presence or absence of EGFR mutations.
Phase 3 trials have shown the advantages of using crizotinib to treat NSCLC in patients who are fluorescence in situ hybridization positive [Shaw A et al. N Engl J Med 2013]. Guidelines recommend ALK testing for NSCLC patients with an adenocarcinoma component and the use of crizotinib in those patients testing positive [Lindeman NI et al. J Thorac Oncol 2013]. Dr. Bunn sees the use of more oncogenic drivers in the future as the cost of testing decreases and the reliability of the tests improve.
Luis Paz-Ares, MD, PhD, Hospital Universitario Virgen del Rocío, Instituto de Investigaciones Biomédicas de Sevilla, Seville, Spain, advocated maintenance therapy for patients with cancer as a way to delay disease progression and improve survival.
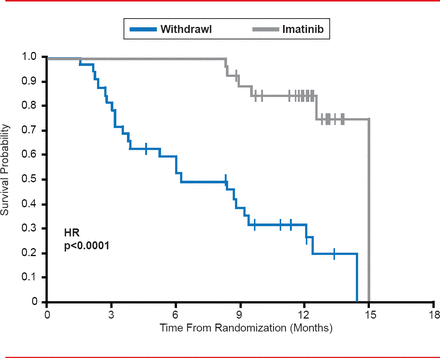
When treatment is withdrawn or interrupted in patients with advanced gastrointestinal stromal tumors (GIST) treated with imatinib, there is a high risk of rapid disease progression (Figure 2) [Le Cesne A et al. Lancet Oncol 2010].
Effects of Imatinib Withdrawal in GIST Patients
Reproduced from Le Cesne A et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncology 2010;11(10):942–949. With permission from Elsevier.
Prof. Paz-Ares recommends using a continuation approach with platinum doublet chemotherapy or a nonplatinum agent or switching to a cytotoxic agent that has not been used previously [Stinchcombe TE et al. J Thorac Oncol 2009]. Outcomes with either protocol are similar, but maintenance therapy following induction therapy should be started before signs of disease progression. Shorter courses are associated with less toxicity than longer, but QoL advantages are similar for both approaches [Smith IE et al. J Clin Oncol 2001].
Both pemetrexed and erlotinib are well tolerated and lead to improved PFS and OS when used as maintenance or switch therapy in patients with NSCLC [Ciuleanu T et al. Lancet 2009; Cappuzzo F et al. Lancet Oncol 2010]. Pemetrexed is effective as maintenance therapy even when previously used during induction therapy but toxicity is moderately elevated, particularly neutropenia and fatigue [Paz-Ares LG et al. Lancet Oncol 2012]. Prof. Paz-Ares noted that if there is a good response to an agent in the induction phase, there is usually no added benefit to switching to a new agent during the maintenance phase.
There are drawbacks to maintenance. It usually does not improve QoL, there are toxicity issues to be considered, and it is not appropriate for patients with the ECOG PS of 2. Also, the expense-benefit cost ratio is yet to be studied. Maintenance therapy is also better for patients with nonsquamous histology. Prof. Paz-Ares said that he is not sure if it should be given to every patient but he does believe that it should be offered as an option for patients whose disease has not progressed after four to six cycles of first-line chemotherapy. As findings are inconclusive at this point, further research is warranted.
- © 2013 MD Conference Express®