Summary
Investigation of patients with mild bleeding disorders might provide novel information on the regulation and role of platelet proteins. It might even identify new targets for prevention of thrombosis. However, gene mutations require phenotypic support to assign causation, according to findings from the observational study, Genotyping and Platelet Phenotyping [GAPP; ISRCTN77951167; UKCRN ID 9858]. This article presents results to date from the study.
- Coagulation Defects
- Purpurea
- Other Hemorrhagic Conditions
- Hematology
- Coagulation Defects
- Purpurea
- Other Hemorrhagic Conditions
Investigation of patients with mild bleeding disorders might provide novel information on the regulation and role of platelet proteins. It might even identify new targets for prevention of thrombosis. However, gene mutations require phenotypic support to assign causation, according to findings from the observational study, Genotyping and Platelet Phenotyping [GAPP; ISRCTN77951167; UKCRN ID 9858]. Steve P. Watson, PhD, University of Birmingham, Birmingham, United Kingdom, presented results to date from the study.
Several factors have contributed to the fact that platelet function disorders are heavily underdiagnosed, including the absence of a “gold standard” point-of-care assay of platelet function and the variable penetrance of bleeding in families with inherited disorders of platelet function. The GAPP study is testing the hypothesis that a proportion of patients who present with excessive bleeding have a previously unrecognized impairment in platelet function that may explain their propensity to bleed in conditions that would not normally be associated with severe bleeding.
The study uses a combination of platelet phenotyping and a combination of targeted and whole exome gene sequencing to identify candidate mutations underlying platelet dysfunction. The effect of a small number of missense mutations discovered in the study on protein function is being investigated through expression studies in immortalized cell lines
To date more than 520 participants, including patients with excessive bleeding suggestive of inherited platelet dysfunction and healthy volunteers, have been recruited to this multicenter study. The main inclusion criteria for patients are patients of any age with excessive bleeding who are willing to participate and are able to provide informed consent. Exclusions include known platelet disorders, such as Glanzmann's thrombasthenia, Bernard Soulier syndrome, Hermansky Pudlak syndrome, and May Hegglin anomaly.
Today, light transmission aggregometry (LTA) is used worldwide for the study of heritable platelet function disorders (PFDs), but interpretation of results is complicated by the feedback effects of adenosine diphosphate (ADP) and thromboxane A(2) [TxA(2)] and the overlap with the response of healthy volunteers [Dawood BB et al. Blood 2012].
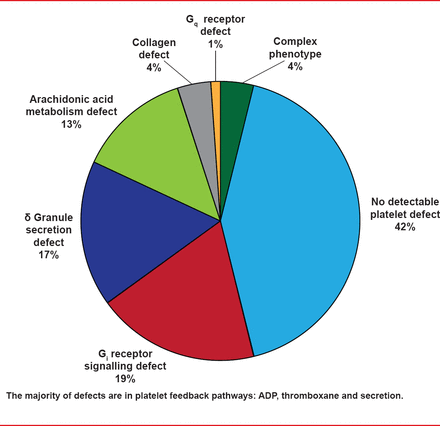
The GAPP study performed lumi-aggregometry on 9 platelet agonists in patients with suspected PFD and in healthy volunteers. Abnormal LTA or adenosine triphosphate (ATP) secretion test results were identified in 58% of patients in the GAPP study. In 84% of these, the patterns of response were consistent with defects in Gi receptor signaling, the TxA(2) pathway, and dense granule secretion. Targeted genotyping identified three participants with function-disrupting mutations in the p2Y (12), ADP, and TxA(2) receptors (Figure 1). Prof. Watson noted that the majority of defects were in platelet feedback pathways: ADP, thromboxane, and secretion.
Classification of Defects
Adapted from Dawood BB et al. Evaluation of participants with suspected heritable platelet function disorders including recommendation and validation of a streamlined agonist panel. Blood 2012;120:5041–5049.
Research illustrates that detailed phenotypic analysis using a rationalized panel of LTA agonists and simultaneously measuring ATP secretion is a powerful tool for the diagnosis of PFDs and in guiding targeted genetic investigations [Watson SP et al. J Throm Haemost 2013].
- © 2013 MD Conference Express®