Summary
This article presents data from the Assessment by a Double Randomization of a Conventional Antiplatelet Strategy Versus a Monitoring-Guided Strategy for Drug-Eluting Stent Implantation and of Treatment Interruption Versus Continuation One Year After Stenting trial [ARCTIC-INTERRUPTION; NCT00827411], demonstrating that patients who do not experience a major cardiac event within 1 year of drug-eluting stent implantation may not require long-term dual antiplatelet therapy.
- Thrombotic Disorders
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Thrombotic Disorders
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
- Interventional Techniques & Devices
Gilles Montalescot, MD, PhD, Institut de Cardiologie, Pitié-Salpêtrière University Hospital, Paris, France, presented data from the Assessment by a Double Randomization of a Conventional Antiplatelet Strategy Versus a Monitoring-Guided Strategy for Drug-Eluting Stent Implantation and of Treatment Interruption Versus Continuation One Year After Stenting trial [ARCTIC-INTERRUPTION; NCT00827411], demonstrating that patients who do not experience a major cardiac event within 1 year of drug-eluting stent (DES) implantation may not require long-term dual antiplatelet therapy (DAPT).
Uncertainty regarding the optimal duration of DAPT poses a challenge in the management of patients following DES implantation [Kandzari DE et al. JACC Cardiol Intv 2009]. International guidelines differ, and in North America, long-term DAPT, for at least 12 months, is recommended in these patients [Feres F et al. JAMA 2013; Levine GN et al. Circulation 2011], while European guidelines recommend at least 6 months DAPT [Wijns W et al. Eur Heart J 2010]. To date, DAPT extended beyond 12 months has been considered to favor clinical outcomes in selected patients [Feres F et al. JAMA 2013; Levine GN et al. Circulation 2011; Wijns W et al. Eur Heart J 2010]. However, evaluation of pooled data from several randomized studies has suggested that extended DAPT offers no ischemic benefit to patients, and appears to increase the incidence of major bleeding events [Cassesse S et al. Eur Heart J 2012].
ARCTIC-INTERRUPTION was a prospective, randomized trial that was designed to compare the safety and clinical impact of 12 months versus 18 to 30 months of DAPT after percutaneous coronary intervention (PCI). The trial was an extension of the original ARCTIC trial, which demonstrated that monitoring platelet function in patients (n=2440) receiving antiplatelet therapy did not improve clinical outcomes [Collet JP et al. N Engl J Med 2012].
Randomization occurred at the end of the first year of follow-up after stenting. The primary endpoint of the ARCTIC-INTERRUPTION trial was the composite of death, myocardial infarction (MI), stent thrombosis, stroke, or urgent revascularization after 1 year. The major secondary endpoint was stent thrombosis or any urgent revascularization.
A total of 1259 patients from the original ARCTIC trial who were free of major events within a year after coronary stenting were included in the study, and re-randomized to either interruption of the DAPT regimen with a switch to single antiplatelet therapy (n=624), or continued DAPT for up to an additional year (n=635). Patients were excluded if they had any primary efficacy or safety endpoints during the first 12 months of follow-up; any new revascularization requiring prolonged DAPT; contraindication to aspirin continuation; or physician or patient decision not to stop thienopyridine at 1 year.
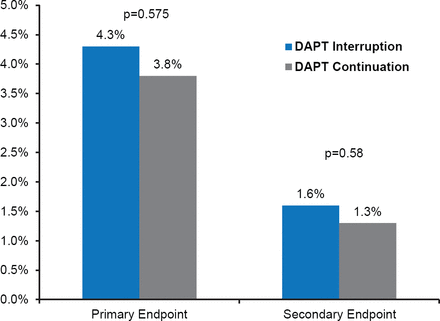
There was no statistically significant difference between the DAPT interruption and DAPT continuation groups with respect to occurrence of the primary endpoint up to 18 months after randomization (4.3% vs 3.8%; HR, 1.17; 95% CI, 0.68 to 2.03; p=0.575), or secondary endpoint (1.6% vs 1.3%; HR, 1.30; 95% CI, 0.51 to 3.30; p=0.58; Figure 1).
Primary and Secondary Endpoints
DAPT=dual antiplatelet therapy.
Major bleeding events occurred more frequently in the DAPT continuation versus the DAPT interruption group, although this difference was not statistically significant (1.1% vs 0.2%; HR, 0.15; 95% CI, 0.02 to 1.20; p=0.073). A significant difference was found, however, with respect to major or minor bleeding events (1.9% vs 0.5%; HR, 0.26; 95% CI, 0.07 to 0.91; p=0.035).
Prof. Montalescot concluded that in patients who have not experienced a major adverse event within the first year after stent implantation, prolonged continuation of DAPT beyond this time does not provide additional clinical benefit in protecting against ischemia. Additionally, longer-term DAPT may increase the risk of bleeding events.
- © 2013 MD Conference Express®