Summary
Patients undergoing treatment for cancer are at higher risk for cardiovascular disease (CVD), in part because of common risk factors. This article discusses how reduced functional status following cancer treatment can lead to shortened survival in cancer and CVD.
- Breast Cancer
- Prevention & Screening
- Thrombotic Disorders
- Adjuvant/Neoadjuvant Therapy
- Oncology
Patients undergoing treatment for cancer are at higher risk for cardiovascular disease (CVD), in part because of common risk factors. Susan G. Lakoski, MD, University of Vermont, College of Medicine, Burlington, Vermont, USA, focused on how reduced functional status following cancer treatment can lead to shortened survival in cancer and CVD.
In a study of women with breast cancer, after 10 years following cancer treatment, women had an equal chance of dying from either breast cancer or CVD [Patnaik JL et al. Breast Cancer Res 2011]. There are multiple direct (surgery, radiation, and systemic therapy) and indirect (inactivity, aging, changes in body composition) factors that can lead to impairments in cardiorespiratory fitness (CRF) in cancer patients [Jones LW et al. J Am Coll Cardiol 2007].
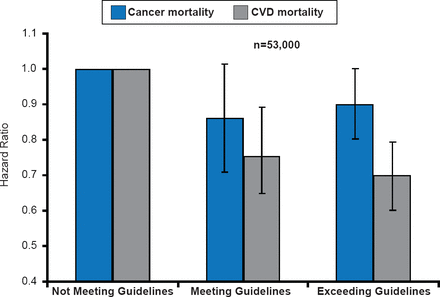
CRF is lowest in the patients treated with multimodal adjuvant therapy [Lakoski SG et al. Breast Cancer Res Treat 2013] and is accompanied by ectopic fat, dyslipidemia, insulin resistance, and hypertension [Van Gaal LC et al. Nature 2006]. The best treatment approach to alter this decline in fitness is physical activity. Guidelines for physical activity for patients undergoing cancer treatment are identical to those for all individuals—strength training ≥2 days/week, 150 minutes of moderate physical activity/week, or 75 minutes of vigorous physical activity/week. Patients meeting or exceeding these guidelines have experienced linear reductions in CVD mortality but inconsistent and limited decreases in cancer mortality (Figure 1)[Barlow CE et al. AHA 2013 (abstr SS-A-11377)].
Effects of Physical Activity Guidelines on Cancer and CVD Mortality: The Cooper Center Longitudinal Study
Reproduced with permission from SG Lakoski, MD.
Dr. Lakoski suggests CRF testing to assess safety and readiness for exercise, determine exercise intensity for patients and compare CRF level with healthy age-sex matched individuals. Optimal fitness level and timing of exercise interventions for cancer patients remains undefined.
Neal L. Weintraub, MD, Medical College of Georgia at Georgia Regents University, Augusta, Georgia, USA, focused his presentation on radiation-induced CVD, which is common in cancer survivors and can occur decades after treatment with an increasing likelihood of a cardiac event with increasing doses of radiation [Darby SC et al. N Engl J Med 2013; Jaworksi C et al. J Am Coll Cardiol 2013].
The increase starts within the first 5 years following radiotherapy and can continue into the third decade after exposure. Women with pre-existing cardiac risk factors are at greater risk of CVD from radiotherapy [Darby SC et al. N Engl J Med 2013]. Although the pericardium is most commonly involved, radiation can induce aortic valve disease, vascular disease, stroke, and baroreflex failure. Radiation-induced CVD is a dynamic process involving a series of cascading events including oxidative stress, activation of nuclear transcription factors, inflammatory cytokines, adhesion molecules, inflammatory cell recruitment, and foam cell formation. Patients need to be evaluated and managed based on underlying cardiac risk factors, patient-, disease- and treatment-specific factors. Dr. Weintraub believes that interventions affecting chronic oxidative stress, fibrosis, and inflammation can modify outcomes from radiation treatment.
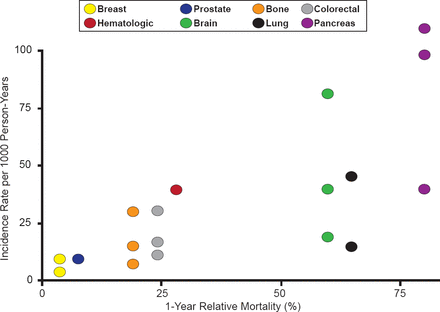
According to Alfonso Tafur, MD, University of Oklahoma, Health Sciences Center, Oklahoma City, Oklahoma, USA, venous thromboembolism (VTE) is a leading cause of death in patients with cancer [Khorana AA et al. J Thromb Haemost 2007]. There is a strong association between types of cancer with worse mortality and those with the highest incidence of VTE (Figure 2) [Timp J et al. Blood 2013].
Incidence Rate of VTE Versus Cancer Type Mortality
Reproduced with permission from A Tafur, MD.
The annual incidence of VTE in patients receiving chemotherapy is estimated at 11%, but it can climb to 20% or higher [Haddad TC, Greeno EW. Thromb Res 2006]. Patients with stomach, lung, brain and pancreatic cancers, have the highest rates of VTE and increased risk of mortality [Timp JF et al. Blood 2013; Horsted F et al. PLoS Med 2012]. Incident VTE however, may not be predicted solely by the cancer type as many other variables are involved, including: chemotherapy, platelet activation, indwelling catheters, endothelium changes, etc. VTE prevention with heparin is effective in patients with solid tumors [Phan M et al. J Throm Thrombolysis 2013]. It is speculated that thromboprophylaxis in cancer patients might improve prognosis and quality of life by preventing thrombotic events, but outpatient primary chemoprophylaxis is not currently advocated in the national guidelines due to lack of adequate data determining long-term safety and mortality benefit [Streiff M et al. J Natl Compr Canc Netw 2013; Lyman G et al. J Clin Oncol 2013]. Risk stratification tools are needed to determine which patients may benefit the most from primary outpatient VTE chromoprophylaxis. The best validated model to date to predict cancer associated thrombosis was developed by Khorana et al. [Blood 2008]. The Khorana score uses five variables: site of cancer, platelet count, hemoglobin and/or use of erythropoiesis-stimulating agents, leukocyte count, and body mass index of ≥35 kg/m2 to predict which patients are at highest risk for incident thrombosis. A Phase 3 randomized trial to evaluate the utility of primary prevention among patients with high VTE risk is currently ongoing [NCT00876915]. In addition, the ongoing discovery of novel biomarkers associated with incident VTE, will likely help us build better prediction scores in the future.
Patients who develop VTE, have a lower likelihood of thrombosis recurrence with no increased risk of bleeding if treated with low-molecular weight heparin (LMWH) compared with warfarin [Lee AYY et al. N Engl J Med 2003]. Therefore, LMWH is currently the preferred treatment for cancer associated VTE. To date, the data evaluating novel anticoagulants in the specific setting of active cancer is limited and such agents should not be used as primary treatment option.
The unlabeled use of trastuzumab for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer was the topic of discussion by Richard Steingart, MD, Memorial Sloan-Kettering Cancer Center, New York, New York, USA. The combined results from two trials in women with surgically removed HER2-positive breast cancer treated with trastuzumab showed significant (p=0.0001) improvement in disease-free survival at 4 years [Romond EH et al. N Engl J Med 2005].
Although there is some risk of cardiac dysfunction with the addition of trastuzumab to adjuvant chemotherapy in these patients, the benefits of outweigh the risks, and cardiac toxicity, if it develops, can be managed [Romond EH et al. J Clin Oncol 2012]. Assessment of left ventricular ejection fraction (LVEF) prior to initiation of trastuzumab and at regular intervals during treatment is recommended [Herceptin (trastuzumab) Highlights of Prescribing Information. South San Francisco, CA: Genentech; 2013 (rev)]. The standard of care is 12 months of trastuzumab treatment [Pivot X et al. Lancet Oncol 2013]. ACE inhibitors are recommended for HER2 patients with LVEF <40% with no signs and symptoms of heart failure (asymptomatic LV dysfunction) [Heart Failure Society of America. J Card Fail 2010].
Many chemotherapy agents that are routinely used have been associated with cardiotoxicity. Apostolia M. Tsimberidou, MD, PhD, University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA, discussed the various cancer therapies and the cardiac dysfunction associated with their use. The use of agents such fluorouracil, interleukin-2, and sorafenib are associated with ischemia. QT prolongation associated with arrhythmias can result from thalidomide and vandetanib use. LV dysfunction is seen with anthracyclines, trastuzumab, and anti-vascular endothelial growth factor drugs such as sunitinib, bevacizumab, and pazopanib.
Changes in the management of cardiotoxicity in cancer patients receiving chemotherapy, improved patient screening and monitoring, identifying patients at increased risk, and establishing standardized procedures and decision support systems, have helped improve outcomes.
- © 2013 MD Conference Express®