Summary
Intravenous tissue plasminogen activator (IV-tPA) is the treatment of choice for acute ischemic stroke, but it is not effective in >50% of patients with large artery occlusion. This article discusses endovascular therapy (EVT) as a revascularization option for these patients, as well as provides update on aneurysms, arteriovenous malformation, and dural arteriovenous fistula EVT.
- Cerebrovascular Disease
- Interventional Techniques & Devices
- Neurology
- Cerebrovascular Disease
- Interventional Techniques & Devices
- Cerebrovascular Disease
Intravenous tissue plasminogen activator (IV-tPA) is the treatment of choice for acute ischemic stroke, but it is not effective in >50% of patients with large artery occlusion. Dileep Yavagal, MD, University of Miami, Miami, Florida, USA, suggested that endovascular therapy (EVT) may offer a revascularization option for these patients.
The Interventional Management of Stroke 3 (IMS-3) and SYNTHESIS Expansion trials [Broderick JP et al. N Engl J Med 2013; Ciccone A et al. N Engl J Med 2013] failed to show a reduction in 90-day modified Rankin Scale score when comparing IV-tPA alone to EVT (alone or in combination with IV-tPA) among patients with moderate to severe acute ischemic stroke. A subgroup analysis of the IMS-3 trial showed that patients with a National Institutes of Health Stroke Scale score >20 who received IV-tPA within 2 hours of stroke onset might benefit from EVT. There was also a benefit of IV-tPA plus EVT in the subgroup of patients with larger artery occlusions who received tissue plasminogen activator within 2 hours of stroke onset [Demchuk A. ISC 2013].
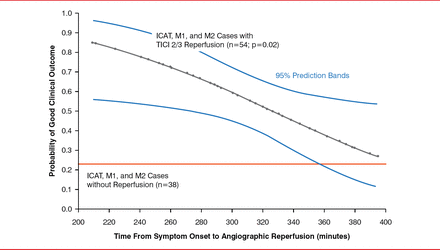
The IMS-3 also showed that complete or near-complete recanalization was associated with improved outcomes. In the IMS-3, when recanalization was ≥50%, the proportion of patients having a good outcome was 47% (ie, thrombolysis in cerebral infarction [TICI] 2b), but when complete recanalization was achieved (ie, TICI 3), the rate increased to ∼70%. The IMS-3 also indicated that time from stroke onset to procedure termination is as critical with EVT as with intravenous therapy. Every 30-minute delay in reperfusion was associated with a 14% relative reduction in probability of good clinical outcome (modified Rankin Scale, 0 to 2). There was apparent benefit when reperfusion therapy was longer than 4.5 hours (Figure 1) [Khatri P et al. Neurology 2009].
Reperfusion Time Following Endovascular Therapy and Stroke Outcome Defined by Modified Rankin Scale
ICAT=internal carotid artery terminus; M1=middle cerebral artery main stem; M2=superior division of middle cerebral artery; TICI=thrombosis in cerebral infarction.
Reproduced from Khatri P et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology 2009;73(13):1066–1072. With permission from Lippincott Williams & Wilkins, Inc.
Second-generation flow restoration (FR) devices (Solitaire) have achieved substantially better angiographic, safety, and clinical outcomes versus first-generation (Merci Retriever) devices in patients with acute ischemic stroke. In the SWIFT trial [Solitaire FR With the Intention for Thrombectomy], use of the Solitaire flow restoration device compared with the Merci device led to more patients with successful recanalization (68.5% vs 30.2%; p<0.0001) as well as good neurologic outcomes at 90 days (58.2% vs 33.3%; p=0.017) [Saver JL et al. Lancet 2012].
New EVT clinical trials are examining thrombus length and newer retriever devices. According to one trial, among patients with acute middle cerebral artery stroke, IV-tPA has little potential to recanalize occluded vessels if thrombus length exceeds 8 mm [Riedel CH et al. Stroke 2011]. The direct aspiration first-pass technique, which is used with a large-bore aspiration catheter as the primary method for vessel recanalization alone, has shown to be successful in 75% (28 of 37) of cases [Turk AS et al. J Neurointerv Surg 2014]. Last, progress is being made in balancing time delays with rapidly effective neuroimaging selection protocols.
Dr. Yavagal followed the above presentation with an update on carotid and intracranial stenting. In CREST [Carotid Revascularization Endarterectomy Versus Stent Trial], primary outcomes with carotid stenting were equivalent to those of carotid endarterectomy (7.2% vs 6.8%; HR 1.11; 95% CI, 0.81 to 1.51; p=0.51). During the periprocedural period, there was higher risk of stroke with stenting and higher risk of myocardial infarction with endarterectomy [Mantese VA et al. Stroke 2010].
The 2011 Carotid Revascularization Guidelines state that patients with average or low surgical risk, nondisabling ischemic stroke within 6 months, and a reduction of the ipsilateral internal carotid artery lumen >70% should undergo endarterectomy. Stenting is indicated as an alternative to endarterectomy. Selection of asymptomatic patients should be guided by assessment of comorbid conditions, life expectancy, and other individual factors. The benefits for asymptomatic patients older than 70 years are smaller.
Intracranial atherosclerosis is the most common cause of stroke worldwide. Patients treated with aggressive medical therapy (AMT) continue to have a significant rate of recurrent stroke (12.2% per year). In the SAMMPRIS trial [Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis], patients with a recent transient ischemic attack or stroke attributed to stenosis of 70% to 99% of the diameter of a major intracranial artery were randomized to aggressive AMT alone or AMT plus percutaneous transluminal angioplasty and stenting with the use of the Wingspan stent system. AMT was found to be superior to angioplasty and stenting because of the significantly (p=0.002) lower risk of stroke [Chimowitz MI et al. N Engl J Med 2011]. The most common causes of periprocedural strokes in SAMMPRIS were wire perforation, reperfusion hemorrhage, and perforator infarct. Unless safer devices are developed, these risks would limit percutaneous transluminal angioplasty and stenting to a small subset of patients [Fiorella D et al. Stroke 2012].
Sam O. Zaidat, MD, MS, Medical College of Wisconsin/Froedtert Hospital, Milwaukee, Wisconsin, USA, provided an update on aneurysms, arteriovenous malformation (AVM), and dural arteriovenous fistula EVT.
Unruptured cerebral aneurysms are present in 3.6% to 6% of the general population. Multiple aneurysms develop in 30% of cases. Major risk factors include being older than 50 years, female, or a cigarette smoker, as well as having a family history of intracranial aneurysms or polycystic kidney disease. Cerebral aneurysms are classified as saccular, fusiform, or dissecting; 90% are saccular. Symptoms include subarachnoid hemorrhage (first symptom in 58% of patients); severe headaches sometimes associated with loss of consciousness, nausea, and vomiting; focal neurologic deficits; or meningismus. One half of patients present with milder symptoms caused by a warning leak before full rupture of the aneurysm.
EVT is safe in both ruptured and unruptured aneurysms and can be used to treat aneurysms >5 mm on the basis of age, shape, history of other aneurysms, family history, and history of a superior hypophyseal artery aneurysm. Smaller aneurysms should be monitored annually.
Investigators of the Unruptured Cerebral Aneurysm Study recently reported that patients with saccular aneurysms ≥3 mm who initially presented with only slight disability had an annual rupture rate of 0.95% (95% CI, 0.79 to 1.15), which increased with aneurysm size. Compared with other aneurysms, those with a daughter sac (ie, an irregular protrusion of the wall of the aneurysm; HR, 1.63; 95% CI, 1.08 to 2.48) and those in the posterior and anterior communicating arteries (HR, 1.90 [95% CI, 1.12 to 3.21] and 2.02 [95% CI, 1.13 to 3.58], respectively) were more likely to rupture [Morita A et al. N Engl J Med 2012].
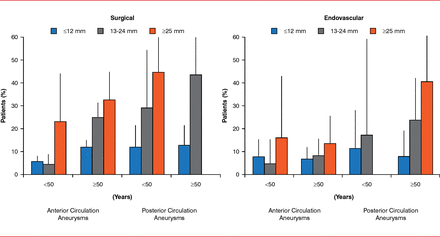
Aneurysms can be treated with coiling, neck remodeling, intracranial aneurysmal stents, flow diverters, and liquid embolization. Extra luminal or surgical clipping is well established but invasive, with a high rate of complications. Endoluminal or endovascular therapy is less invasive, with significantly reduced perioperative risk but with increased risk of recurrence and retreatment. Authors of the 2003 International Study of Unruptured Intracranial Aneurysms reported less procedural intracranial hemorrhage and cerebral infarction with EVT [Wiebers DO et al. Lancet 2003] based on aneurysm size and location and patient age (Figure 2).
Percentage of Patients With Complications Following Surgical Versus Endovascular Treatment
Reproduced from Wiebers DO et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003;362(9378):103–110. With permission from Elsevier.
Dural arteriovenous fistulas are rare vascular anomalies formed by an abnormal connection between arteries and veins within the dura mater. They commonly have multiple feeders to the fistula site with multiple draining veins. However, there is usually a single point of connection between the arterial and venous systems. Dural arteriovenous fistulas present with pulsatile bruit or tinnitus, headache, visual impairment, and papilledema. Grades are 1 to 3 (Borden classification). Grade 1 can be followed unless disabling symptoms or bleeding occur, while grades 2 and 3 or any cortical drainage may require therapy, which includes an endovascular approach with embolization to the feeding artery or the draining veins to close the fistulas (ie, connections). Risk factors for dural arteriovenous fistula are trauma or dural sinus thrombosis.
Cerebral AVMs are one of the leading causes of intracerebral hemorrhage in the young. They present with headaches, seizures, or focal neurologic deficits. Intracranial hemorrhage accounts for as many as 50% of initial presentations. High-risk AVMs need to be treated, usually with a multimodal approach. A ruptured low-grade AVM requires treatment with embolization (effective in 15% to 60% of cases), surgical resection, radiosurgery, or multimodality approach. For an unruptured AVM, treatment depends on grade and accessibility. In ARUBA [A Randomized Trial of Unruptured Brain Arteriovenous Malformations], the risk of death or stroke was significantly lower in patients who received medical management when compared with those who received interventional therapy (HR, 0.27; 95% CI, 0.14 to 0.54) [Mohr JP et al. Lancet 2014].
- © 2014 MD Conference Express®