Summary
The American Heart Association (AHA)/American College of Cardiology (ACC) clinical practice guidelines for chronic heart failure were updated for 2013 including expanded indications for cardiac resynchronization therapy (CRT), the use of biomarkers in diagnosis and treatment guidance, emerging agents, cell therapy, and the use of mechanical support. This article discusses the updates to the recommendations of the 2013 AHA/ACC guidelines for CRT.
- Cardiology Genomics
- Heart Failure
- Cardiology Guidelines
- Cardiology Genomics
- Heart Failure
- Cardiology Guidelines
- Cardiology
The American Heart Association (AHA)/American College of Cardiology (ACC) clinical practice guidelines for chronic heart failure (HF) were updated for 2013 including expanded indications for cardiac resynchronization therapy (CRT), the use of biomarkers in diagnosis and treatment guidance, emerging agents, cell therapy, and the use of mechanical support. Lynne Warner Stevenson, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA, discussed the updates to the recommendations of the 2013 AHA/ACC guidelines for CRT [Yancy http://circ.ahajournals.org/content/128/16/e240.full.pdf+html]. The new guidelines endorse CRT for patients with Stage C, HF NYHA Class II or III/IV symptoms with a left ventricular ejection fraction (LVEF) of ≤35%, in sinus rhythm, left bundle-branch block (LBBB) with a QRS duration of ≥150 ms.
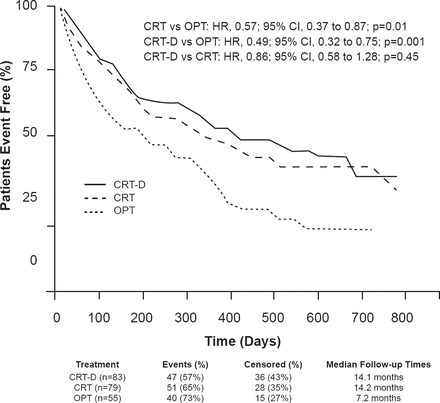
In patients with NYHA symptom Class III to ambulatory IV HF, the addition of CRT to medical therapy appears to improve function and quality of life, as well as decrease hospitalizations by 30% and improve survival by 24% to 36%. In NYHA ambulatory IV patients from the COMPANION trial, the addition of CRT to optimized medical therapy resulted in stable disease for ≥1 month with no hospitalizations related to HF and no IV inotropic therapy (Figure 1) [Lindenfeld J et al. Circulation 2007]. In patients with end-stage HF that required inotropic therapy, CRT resulted in improved ventricular assist device (VAD)- and transplant-free survival compared with inotropic therapy or medical therapy over 60 months [Bhattacharya S et al. J Cardiac Failure 2010].
Benefit of Cardiac Resynchronization Therapy in the COMPANION Trial
CRT=cardiac resynchronization therapy; CRT-D=cardiac resynchronization therapy plus implantable defibrillator; OPT=optimized medical therapy.
Reproduced from Lindenfeld J et al. Effects of cardiac resynchronization therapy with or without a defibrillator on survial and hospitalizations in patients with New York Heart Association class IV heart failure. Circulation 2007;115:204–212. With permission from Lippincott, Williams and Wilkins.
In 4213 patients with mild HF, a systematic review and meta-analysis indicated that although CRT did not significantly reduce mortality (p=0.24), it did decrease HF events and left-ventricular end-diastolic volume while increasing 6-minute walk test and EF (p<0.001 for all) [Santangeli P et al. J Interv Card Electrophysiol 2011].
In two different meta-analyses, the CRT therapy was beneficial only in patients with a QRS ≥150 ms [Stavrakis S et al. J Cardiovasc Electrophysiol 2012; Sipahi I et al. Arch Intern Med 2011]. In the MADIT-CRT trial, patients with a QRS ≥150 msec experienced a significant benefit from CRT therapy (RR, 0.48; 95% CI, 0.37 to 0.63; p<0.001) compared with control therapy; however, patients with a QRS <150 msec did not experience a benefit from CRT therapy (RR 1.06; 95% CI, 0.74 to 1.52; p=0.75) [Moss AJ et al. N Engl J Med 2009]. In the RAFT trial, CRT therapy was beneficial in patients with LBBB versus right bundle-branch block (RBBB) and patients that were paced or had intraventricular conduction delay (IVCD) [Tang AS et al. N Engl J Med 2010]. In addition, patients with atrial fibrillation (AF) and HF did not respond as well as HF patients without AF in two different meta-analyses to CRT therapy [Wilton SB et al. Heart Rhythm 2011; Sharma AK, Heist EK. J Innovat Card Rhythm Manage 2012].
Wilson Tang, MD, Cleveland Clinic, Cleveland, Ohio, USA, presented data on biomarkers used in the diagnosis and treatment of HF. A biomarker is considered to be clinically useful if it can be measured via accurate and reproducible methods, there is a strong and consistent association between the marker and the outcome, and if it is superior to current tests or enhances outcomes of care. Biomarkers are useful adjunct to clinical diagnoses, yet incorporating them into clinical management still requires further refinement. In the 2013 AHA/ACC guidelines, there is a major change in the recommendations regarding biomarker use based on over a decade of clinical trials and experience. Recommended biomarkers include natriuretic peptides for the diagnosis and management of heart failure as well as biomarkers of myocardial injury and fibrosis for risk stratification [Yancy CW et al. Circulation 2013].
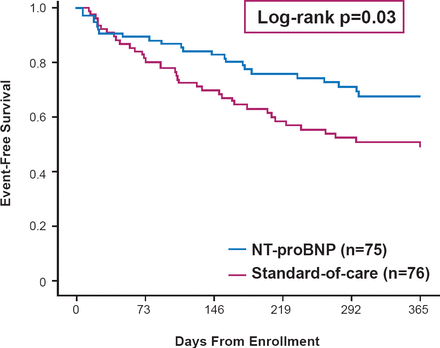
In a meta-analysis of 2686 patients in 12 studies, natriuretic peptide-guided therapy resulted in a greater rate of event free survival compared with the standard of care [Savarese G et al. PLosOne 2013]. The beneficial effects of natriuretic peptide-guided management after hospital discharge has been demonstrated in a single-center study (log-rank p=0.03; Figure 2) [Januzzi Jr. JL et al. J Am Coll Cardiol 2011], and is now being tested in the NIH-funded Guiding Evidence Based Therapy Using Biomarker Intensified Treatment trial [GUIDE-IT]. As a marker of worsening renal function, increases of creatinine <25% relative from the baseline were associated with better event free survival compared with patients with increases of ≥25% [Metra M et al. Eur J Heart Fail 2008], although recent data have also suggested that not all rise in creatinine are detrimental as long as effective decongestion ensue.
Effect of NT-proBNP-Guided Therapy on Event-Free Survival in Heart Failure
NT-proBNP=N-terminal prohormone of brain natriuretic peptide.
Reproduced from Januzzi JL Jr et al. Use of amino-terminal pro-B-natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011;58(18):1881–1889. With permission from Elsevier.
Emerging biomarkers include soluble ST2, which is an interleukin (IL)-1 receptor family member that blocks IL-33 signaling. In one study, the greater the soluble ST2 levels, the lower the rate of transplant-free survival [Ky B et al. Circ Heart Fail 2011]. Another emerging biomarker is galectin-3, which is a soluble β-galactoside-binding lectin that is associated with fibrogenesis and inflammation. In the HF-ACTION study, patients with high levels of both NT-proBNP and galectin-3 had the greatest rate of hospitalization compared with patients that had low levels of NT-proBNP and/or low levels of galectin-3 [Felker GM et al. Circ Heart Fail 2012]. Despite their prospects in risk stratification, how these emerging biomarkers impact current clinical management remains to be determined.
Gary S. Francis, MD, University of Minnesota, Minneapolis, Minnesota, USA, discussed updates to the pharmacologic treatment of HF, including the emerging agent serelaxin.
Serelaxin is a recombinant human protein that is identical to relaxin-2, an endogenous hormone with receptors located in blood vessels, the heart, and the kidneys [Bathgate RA et al. Physiol Rev 2013]. The binding of serelaxin to endothelial or smooth muscle cells results in the release of nitric oxide that causes systemic and renal vasodilation [Schneider MP et al. Ann Rev Pharmacol Toxicol 2007; McGuane JT et al. Hypertension 2011]. Interestingly, relaxin-2 is elevated during the first trimester of pregnancy and is associated with a 30% decrease in systemic vascular resistance, a 20% decrease in renal vascular resistance, a 20% increase in cardiac output and up to 85% increase in renal blood flow. In addition, both systemic and renal vasodilation occurs simultaneously, which is unique to pregnancy [Baylis C et al. Am J Kid Dis 1999; Schrier RW et al. Am J Kid Dis 1987; Jeyabalan A et al. Adv Exp Med Biol 2007].
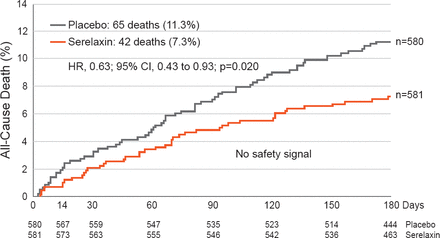
In an intention-to-treat population, treatment with serelaxin resulted in significantly fewer cardiovascular deaths (HR, 0.63; 95% CI, 0.41 to 0.96; p=0.028) and all-cause mortality (HR, 0.63; 95% CI, 0.43 to 0.93; p=0.020; Figure 3) compared with patients that received placebo over 180 days [Teerlink JR et al. Lancet 2013]. Dr. Francis noted that the treatment of HF is evolving slowly and is focused on systolic dysfunction instead of HF with preserved ejection fraction (HFpEF).
Effect of Serelaxin Treatment on All-Cause Death in Patients With Heart Failure
Reproduced from Teerlink JR et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 2013;381(9860):29–39. With permission from Elsevier.
Joshua Hare, MD, University of Miami, Miami, Florida, USA, discussed regenerative medicine in HF. A major regenerative approach to HF is cell therapy for ischemic cardiomyopathy. In this method, mesenchymal stem cells (MSCs) harvested from bone marrow aspirate are expanded in culture. Some methods use cardiac stem cells (CSCs). It is believed that MSCs cause the release of cytokines and growth factors resulting in antifibrotic, immunomodulatory, neoangiogenic, and proregenerative effects. The stem cells can then be delivered to the heart via intravenous, intracoronary, surgical, or transcatheter methods. For example, one delivery method uses a specialized helical needle for stability, has enhanced navigation, and uses contrast imaging to guide delivery of stem cells.
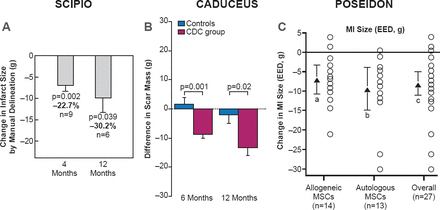
In the SCIPIO, CADUCEUS, and POSEIDON trials, patients that received stem cells as CSCs, cardiospheres, or MSCs experienced a decrease in infarct scar size (Figure 4) [Telukuntla KS et al. J Am Heart Assoc 2013]. In addition, patients in the SCIPIO and POSEIDON trials reported an improvement in quality of life. A decrease in sphericity index in the POSEIDON trial suggests that MSCs can reverse remodeling in patients with HF.
Effect of Cell. Therapy on Infarct Scar Size
CDC=cardiosphere-derived cells; EED=early enhancement defect; MI=myocardial infarction; MSCs=mesenchymal stem cells.
Reproduced from Telekuntla KS et al. The Advancing Field of Cell-Based Therapy: Insights and Lessons From Clinical Trials. J Am Heart Assoc 2013. With permission from Lippincott, Williams and Wilkins.
Lawrence S. Czer, MD, Cedars-Sinai Heart Institute, Los Angeles, California, USA, presented information about mechanical support in HF. Current mechanical support includes extracorporeal membrane oxygenation (ECMO), implantable mechanical circulatory support (MCS), and the total artificial heart.
ECMO is typically used in patients who require an emergency option for biventricular support. In one study, 58% of patients that received ECMO therapy were alive at hospital discharge and another study reported a survival rate of 24% at 5 years. However, it is not widely available, requires perfusion support, can be used for relatively short duration, and its use has associated vascular access complications. Implantable MCS devices are now smaller and more durable with continuos axial and centrifugal flow devices. In a prospective, noncontrolled trial, 281 patients received an implantable MCS, which resulted in a survival rate of 82% at 6 months and 73% at 12 months, as well as an improvement in the 6-minute walk test at 6 months [Pagani FD et al. J Am Coll Cardiol 2009].
An implantable, pulsatile, pneumatic pump, the total artificial heart was approved by the United States Food and Drug Administration in 2004. In a trial of 81 patients with HF, patients who received the total artificial heart, 79% survived until transplant compared with 46% of patients that received medical therapy alone.
Dr. Czer pointed out that mechanical support is effective as a bridge to transplant and 43% of all patients that have received a transplant received MCS while waiting for transplantation [Peura JL et al. Circulation 2012]. Typically, the 1-year survival rate of patients awaiting heart transplant due to HF is 23%; however, MCS improves survival, functionality, and quality of life in these patients.
Survival and quality of life have been improved in patients with HF due to advances in CRT, pharmacologic therapy, cell therapy, and mechanical support devices. Importantly, tailoring therapy based on the presence of LBBB, QRS duration, and elevated biomarkers appear to provide provide further benefit. In addition, emerging therapies have the potential to expand treatment choices for patients with HF.
- © 2013 MD Conference Express®