Summary
This article presents an overview of what is known about insulin and cancer. Specific results from the Northern European Database Study, the Kaiser Permanente Collaboration, as well as implications for practice and future research are also discussed.
- Hormone Therapy
- Insulin
- Breast Cancer
John Buse, MD, PhD, University of North Carolina School of Medicine, Chapel Hill, North Carolina, USA, presented an overview of what is known about insulin and cancer. A MEDLINE search for insulin and cancer on May 22, 2012, found 25,543 articles.
Hemkens et al. [Hemkens LG et al. Diabetologia 2009] were the first investigators to report that patients who used higher doses of glargine had an increased risk of cancer of all forms. However, results from other studies have been inconsistent.
A combined analysis of 31 randomized controlled trials (mostly of 6-month duration) found no difference in cancer occurrence between insulin glargine and comparative groups (mostly neutral protamine Hagedorn, or NPH, insulin) [Home PD, Lagarenne P. Diabetologia 2009]. Data from a long-term randomized trial in patients with type 2 diabetes [Rosenstock J et al. Diabetologia 2009] also found no evidence of any difference in the rate of benign or malignant tumor development with insulin glargine versus NPH insulin.
Chang et al. [Chang C-H et al. PLoS One 2011] found a dose-dependent increase in cancer risk for treatment with glargine compared with human insulin (p<0.0001): the adjusted HR was 1.09 (95% CI, 1.00 to 1.19) for a daily dose of 10 IU, 1.19 (95% CI, 1.10 to 1.30) for a daily dose of 30 IU, and 1.31 (95% CI, 1.20 to 1.42) for a daily dose of 50 IU. The researchers found no increased risk for aspart (p=0.30) or lispro (p=0.96) compared with human insulin.
Studies in Sweden and Scotland suggest that women who used insulin glargine alone had a significantly higher risk of breast cancer compared with users of other types of insulin, whereas this increased risk was not observed among those who received insulin glargine in combination with other insulin [Jonasson JM et al. Diabetologia 2009; Colhoun HM et al. Diabetologia 2009].
More recent reports have found no increased cancer risk from glargine exposure (eg, the Northern European Study of Insulin and Cancer and the Kaiser Permanente California Cohort Study).
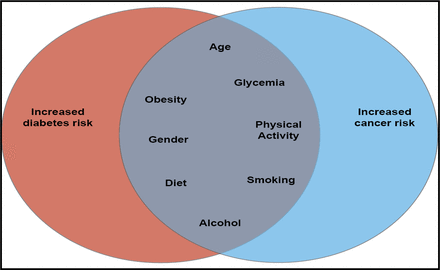
Common risk factors for diabetes and cancer include age, glycemia, obesity, physical activity, gender, smoking, diet, and alcohol use [Giovannuci E et al. Diabetes Care 2010] (Figure 1). However, it has yet to be determined which of these factors is associated with cancer and what potential mechanisms might mediate these associations.
Common Risk Factors for Diabetes and Cancer.
Reproduced with permission from the American Diabetes Association. Giovannuci E et al. Diabetes Care 2010.
Clayton et al. investigated the potential involvement of growth hormone (GH), insulin-like growth factor (IGF-1), and insulin on tumor promotion and progression. The authors endorsed long-term surveillance for cancer incidence in all populations that have been exposed to increased levels of GH [Clayton PE et al. Nat Rev Endocrinol 2011].
Agin et al. [Agin A et al. Diabetes Metab 2007] assessed in vitro glargine blood biotransformation and its inter-individual variability. Outcomes showed that inter-individual variability of glargine biotransformation is noteworthy. However, the bioactivity of glargine metabolites must be assessed before clinical observations of glargine inter-individual variability and its metabolism can be correlated.
The pharmacological bioactivity of glargine metabolites has been suggested by Kuerzel et al. [Kuerzel GU et al. Curr Med Res Opin 2003] but is not yet clearly demonstrated.
Sommerfeld et al. [Sommerfeld MR et al. PloS One 2010] characterized the glargine metabolites in vitro with regard to their insulin receptor (IR) and IGF-1 receptor (IGF1R) binding and signaling properties, as well as their metabolic and mitogenic activities. Findings strongly supported the idea that insulin glargine metabolites contribute with the same potency as insulin glargine to blood glucose control but lead to significantly reduced growth-promoting activity.
The Northern European Database Study
Peter Boyle, DSc, FRCP, FRCPS, FMedSci, International Prevention Research Institute, Lyon, France, discussed the results and context of the Northern European Database Study of Insulin and Cancer Risk [Abstract CT-SY13. American Diabetes Association 2012]. Because administrative databases were used in the report, it shares important limitations in common with other studies of this type, such as confounding, missing data; an inability to control for confounders that are not measured; lack of information on exposure time; and no reliable proof of causation.
The study compared the risk of breast cancer in women, prostate cancer in men, and colorectal cancer in men and women who were prescribed insulin glargine versus human insulin and all users of insulin combined. Secondary objectives were to compare the risk of all forms of cancer combined (except nonmelanoma skin cancer but including hematological malignancies) in the study population.
A total of 17,800 cancers of all forms (excluding nonmelanoma skin cancer) were detected in 447,821 insulin users since glargine was introduced in the individual countries. The study generated more than 1.5 million person-years of exposure to insulin, with an average of 3.1 years of follow-up for patients using glargine.
Analyses showed no differences in the risk of all forms of cancer combined, breast cancer, colorectal cancer, or prostate cancer between users of insulin glargine versus other insulins. They also showed no indication of an increased risk for lung or pancreatic cancer with the use of glargine; no difference between the effects of insulin glargine versus other long-acting insulins on cancer risk; no association between metformin and the forms of cancer that were investigated in the study; and no effect of adjusting for confounders, including body mass index (BMI) and tobacco smoking.
The Kaiser Permanente Collaboration
Laurel Habel, PhD, Kaiser Permanente Division of Research, Oakland, California, USA, shared outcomes from the Kaiser Permanente California Cohort Study.
Kaiser Permanente is a prepaid, integrated health care delivery system with approximately 6.8 million members. The main analyses among prevalent users of NPH insulin examined if switching from NPH insulin to glargine is associated with increased risk of cancer. Among new users of insulin, it examined whether or not initiation of glargine versus NPH insulin is associated with an increased risk of cancer.
Cox regression with time-varying medication use was adjusted for confounders and used to analyze data from existing and new insulin users. Follow-up ended at cancer, disenrollment, or the end of the study. Cox regression with no time-varying medication use was adjusted for confounders and used in the secondary new user analysis. Follow-up ended at stopping or switching insulin types, cancer diagnosis, disenrollment, or the end of the study.
Researchers assessed every use and duration of glargine use (<2 and ≥2 years). Potential confounders included HbA1C levels, type 1 or type 2 diabetes, duration of diabetes, other diabetes medications, BMI, race/ethnicity, smoking, income, alcohol use, and cancer screening. No evidence of confounding by these variables was observed.
Outcomes showed that initiating or switching to glargine is not associated with prostate, colorectal, or all cancers combined. According to Dr. Habel, a time-exposure analysis of breast cancer risk showed that those who had been using glargine at baseline had a suggestion of a very modest increase in risk of 1.6 after 24 months. For patients who had been using glargine for at least 2 years after switching from another insulin, there was no increase in risk.
Implications for Practice and Future Research
James B. Meigs, MD, MPH, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA, reviewed data from the United States and Northern Europe and their implications for practice and future research. He discussed past studies and new outcomes data from Inovalon, Kaiser Permanente, the Northern European Database Study, and the Outcome Reduction With Initial Glargine Intervention [ORIGIN; NCT00069784] trial. He mentioned a meta-analysis of 38 studies, in which Boyle [Boyle P. Abstract: 296 World Diabetes Congress, Dubai 2011] found no increased risk of cancer in >1 million person-years of glargine exposure.
Three questions remain: What have we learned about methods of pharmacosurveillance for safety? Is it safe to prescribe glargine insulin? What have we learned about insulin-associated cancer risk?
The basics of clinical study design underlie the answers: exposure, outcome, and definition of denominator; accounting for confounding, shared risk factors; and limiting bias (ie, missing data) and allocation bias/confounding by indication. Dr. Meigs stressed the need to use data in ways for which they were intended, and that observation does not equal causation.
Registry research is complex and difficult, and even the largest data resources have limitations in ascertaining exposure with adequate length of follow-up. However, the preponderance of data show that it is safe to prescribe glargine insulin, and the benefits of this therapy outweigh potential harm.
Dr. Meigs concluded that no short-term cancer risk is associated with glargine insulin therapy; long-term follow-up of approximately 10 years among new insulin users without cancer is needed (but difficult to obtain); and insulin-cancer risk research is difficult to conduct correctly.
- © 2012 MD Conference Express®