Summary
Genetic factors may be responsible for some of the inter-individual variability in dabigatran exposure, according to findings from the Randomized Evaluation of Long-Term Anticoagulation Therapy [RE-LY] Genetics study. The RE-LY trial demonstrated that dabigatran 150 mg BID was superior to warfarin, while the 110 mg dose was noninferior to warfarin in the reduction of stroke in patients with atrial fibrillation [Connolly SJ et al. N Engl J Med 2009].
- Thrombotic Disorders
- Cardiology Clinical Trials
- Cardiology Genomics
Genetic factors may be responsible for some of the inter-individual variability in dabigatran exposure, according to findings from the Randomized Evaluation of Long-Term Anticoagulation Therapy [RE-LY] Genetics study. The RE-LY trial demonstrated that dabigatran 150 mg BID was superior to warfarin, while the 110 mg dose was noninferior to warfarin in the reduction of stroke in patients with atrial fibrillation [Connolly SJ et al. N Engl J Med 2009]. The lower dose was associated with less major bleeding when compared with warfarin, while the higher dose (150 mg) had a similar rate of major bleeding.
Dabigatran etexilate is an oral prodrug that is rapidly converted by esterases (carboxylesterase-1 [CES1]) to the active agent dabigatran, explained Guillaume Pare, MD, McMaster University, Hamilton, Ontario, Canada, who presented the findings of the study. CES1 is a serine esterase that can activate or deactivate various drugs. Prof. Pare and colleagues hypothesized that genetic variability in the pathways required for bioactivation of dabigatran might be responsible for some of the 30% variability in dabigatran exposure.
In the first phase of the study, a genome-wide analysis (551,203 markers) was performed on biologic samples from 1490 patients of European ancestry enrolled in the RE-LY trial randomized to dabigatran to identify the genetic determinants of peak and trough concentrations of dabigatran. Another 807 patients from RE-LY treated with warfarin also underwent genotyping. Identified genetic determinants were tested for their association with efficacy and safety outcomes in an overlapping sample of 1694 patients. The primary efficacy endpoint was stroke or systemic embolism, and the primary safety endpoint was any bleeding (minor or major).
Genome-wide analysis demonstrated 2 variants associated with peak concentration of dabigatran, one at the ABCB1 locus (rs4148738) and one at the CES1 locus (rs8192935). The ABCB1 polymorphism was associated with a 12% increase in peak concentration per minor allele, while the CES1 polymorphism was associated with a 12% decrease in peak concentration per minor allele (p=8.2×10−8 and p=3.2×10−8, respectively). Two variants were associated with trough concentration: rs4580160 at the CES1P2 locus and rs2244613 at the CES1 locus. The CES1 polymorphism had a significant effect, with a 15% decrease in trough concentration per minor allele (p=1.2×10−8).
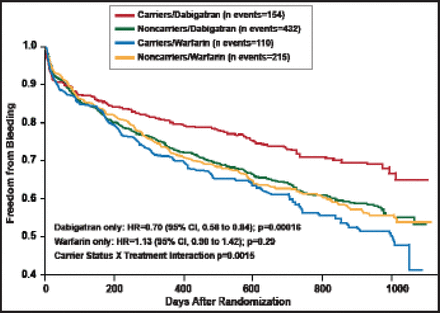
None of these genetic determinants had a significant association with efficacy, but the CES1 rs2244613 variant did have a significant association with bleeding (Table 1). A significant interaction by treatment arm was also noted: the odds of bleeding with dabigatran decreased 33% for carriers of the CES1 rs2244613 polymorphism, while there was no impact of this genetic variant on bleeding for those receiving warfarin (Figure 1). Approximately one third of Europeans are carriers of this polymorphism, said Prof. Pare.
Association of Significant Genetic Determinants with Efficacy and Safety.
Freedom from Bleeding According to CES1 rs2244613 Carrier Status in the RE-LY Study.
Reproduced with permission from G. Pare, MD.
This analysis provides evidence supporting the idea of dose modification of dabigatran based on genotype but requires additional study.
- © 2012 MD Conference Express®