Summary
For patients with severe aortic stenosis, open-heart surgical valve replacement remains the current clinical standard with excellent long-term outcomes [Schoenhagen P et al. J Cardiovasc Comput Tomogr 2011]. For those with severe aortic stenosis who are considered too high risk for the traditional approach, transcatheter aortic valve implantation (TAVI) has proven to be a viable treatment option [Sinning JM et al. Methodist Debakey Cardiovasc J 2012]. This article discusses the use of aortic multislice computed tomography for TAVI screening.
- Valvular Disease
- Cardiology
- Interventional Techniques & Devices
For patients with severe aortic stenosis, open-heart surgical valve replacement remains the current clinical standard with excellent long-term outcomes [Schoenhagen P et al. J Cardiovasc Comput Tomogr 2011]. For those with severe aortic stenosis who are considered too high risk for the traditional approach, transcatheter aortic valve implantation (TAVI) has proven to be a viable treatment option [Sinning JM et al. Methodist Debakey Cardiovasc J 2012].
With the traditional surgical approach, direct inspection of the valve is possible [Ewe SH et al. Int J Cardiovasc Imaging 2011]. However, transcatheter valvular procedures are characterized by lack of exposure of the operative field [Schoenhagen P et al. J Cardiovasc Comput Tomogr 2011]. This makes preprocedural imaging of the anatomy of the aortic or mitral valve and their spatial relationships crucial to select the most appropriate device or prosthesis and plan the percutaneous procedure [Ewe SH et al. Int J Cardiovasc Imaging 2011].
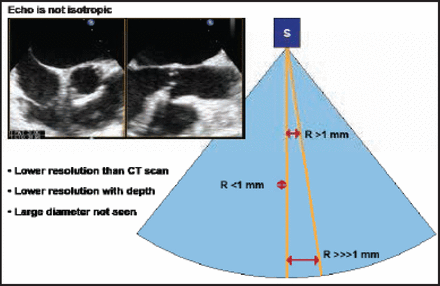
Bernard Chevalier, MD, Institut Cardiovasculaire Paris Sud (ICPS), Massy, France, discussed the use of aortic multislice computed tomography (MSCT) for TAVI screening. Topics covered included the CT scan, aorta, coronary ostia, bicuspidity, aortic calcification, aortic valve area, the annulus plan for view selection during TAVI, annulus size, and the actual shapes of the annulus and crown. He emphasized how echocardiography only shows the anteroposterior diameter and the potential for error if this measurement alone is used. He described how echocardiography is not isotopic compared with the CT scan (Figure 1). Resolution with echocardiography is less than that of a CT scan, with a lower depth resolution and an inability to cover a large diameter. Conversely, CT scans are 3D and isotropic, with a 0.5-mm resolution in all directions. These features may help determine the optimal view, said Prof. Chevalier.
Echocardiography Is Not Isotropic.
Reproduced with permission from B. Chevalier, MD.
Prof. Chevalier also highlighted the clinical impact of moderate- to high-grade aortic regurgitation (AR) and described the ICPS experience with AR, annulus sizing, and predictors of AR ≥2. Aortic valve regurgitation has been considered a potential contributor to morbidity and mortality after TAVI. The reported prevalence of moderate and severe AR after TAVI is 6% to 21%, which is considerably higher than after a surgical valve replacement [Gotzmann M et al. Am Heart J 2012]. Among 175 ICPS patients, post-TAVI aortic regurgitation ≥2 occurred in significantly fewer patients whose surgery was CT-guided (n=27; 15.4%) as opposed to transesophageal echocardiography (TEE)-guided (n=42; 24%; p=0.04).
Procedural Success
Pires de Morais et al. [Rev Port Cardiol 2011] used MSCT to select candidates for transcatheter aortic valve replacement, procedural support, and postinterventional follow-up. The authors found MSCT to be an essential imaging tool in the selection and exclusion of candidates for TAVI; the evaluation of coronary anatomy and the relationship of the coronary ostia with the aortic valve structure; and the accurate analysis of the valve annulus and aortic root, left ventricular outflow tract, aorta, and peripheral vascular access routes. They also described MSCT as central to the choice of appropriate prosthesis size.
Procedural success depends on precise valve positioning in the 3D space of the aortic annulus and root [Gurvitch R et al. J Am Coll Cardiol Intv 2010]. Incorrect positioning may result in valve embolization, severe aortic regurgitation, coronary obstruction, heart block, or impaired left ventricular function.
MSCT has shown utility in TAVI [Wood DA et al. Am J Cardiol 2009; Tops LF et al. J Am Coll Cardiol Img 2008; Ng AC et al. Circ Cardiovasc Imaging 2010]. Prof. Chevalier reports high resolution (0.5 mm) in the 3 axes (X, Y, Z), and true 3D imaging that is optimal for calcified structures and prosthetic materials.
Feuchtner et al. [J Am Coll Cardiol 2006] found that the approach may not only be useful in quantifying valve calcification but also in determining aortic valve area. According to Choo and Steeds [Br J Radiol 2011], MSCT is developing a particularly prominent role in planning TAVI, where it can deliver accurate measurements of the extent of calcification, as well as the annulus and the angulation between the left ventricular apex and the aortic root.
Gurvitch et al. [J Am Coll Cardiol Intv 2010] assessed whether MSCT could predict optimal angiographic projections for visualizing the plane of the native valve and facilitate accurate positioning during transcatheter aortic valve implantation in 20 patients who underwent MSCT before TAVI. Outcomes showed that preprocedural MSCT can predict optimal angiographic deployment projections for implantation of transcatheter valves. An ideal deployment angle curve or “line of perpendicularity” can be generated, improving the accuracy of valve deployment and outcomes.
Multislice Computed Tomography and the Aortic Annulus
In a study comparing transthoracic echocardiography or TEE measurement of the annulus, MSCT identified that the aortic annulus was commonly eccentric and often oval [Wood DA et al. Am J Cardiol 2009].
According to Prof. Chevalier, accurate knowledge of the size of the annulus is very important: overestimation risks annulus rupture and possible valve dysfunction; underestimation increases the risks of embolization and AR. He reminded the audience that the annulus is a crown with 3 branches, and the crown is not circular. It has a variable orientation (≤30 degrees), a small diameter that is often anteroposterior, a large diameter that is grossly lateral, and variability between the 2 diameters (4 to 5 mm, and 1 to 8 mm).
- © 2012 MD Conference Express®