Summary
Untreated septic shock is usually fatal within 24 to 36 hours. Clinical trials with anti-inflammatory agents in patients with sepsis are based on the assumption that the pathogenesis of sepsis is primarily driven by excessive proinflammatory activity of the cytokine network even though the triggering infection may have been eliminated by appropriate antimicrobial therapy [van der Poll T, van Deventer SJ. Infect Dis Clin North Am 1999]. It has been suggested that the failure of these trials to show clinical benefit, in conjunction with recent experimental data, raises doubt about the validity of this assumption.
- Pneumonia
- Bacterial Infections
- Viral Infections
Untreated septic shock is usually fatal within 24 to 36 hours. Clinical trials with anti-inflammatory agents in patients with sepsis are based on the assumption that the pathogenesis of sepsis is primarily driven by excessive proinflammatory activity of the cytokine network even though the triggering infection may have been eliminated by appropriate antimicrobial therapy [van der Poll T, van Deventer SJ. Infect Dis Clin North Am 1999]. Anand Kumar, MD, University of Manitoba, Winnipeg, Canada, suggested that the failure of these trials to show clinical benefit, in conjunction with recent experimental data, raises doubt about the validity of this assumption.
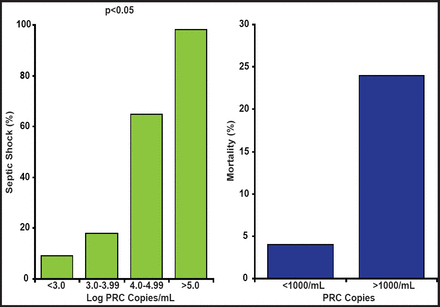
In patients with pneumococcal pneumonia, bacterial load is associated with the likelihood of death and the risk of septic shock [Rello J et al. Chest 2009]. As the log PCR copies of the organism go up, so does the probability of septic shock and death (Figure 1). Many studies have shown that time to antimicrobial therapy is a critical determinant of survival in meningococcal sepsis. However, when the relative impact of blood bacterial load and time to antimicrobial therapy on mortality in patients with meningococcal sepsis is considered, the critical factor is blood bacterial load. This suggests that delays in antimicrobial treatment simply mark the development of a greater bacterial load with delays in therapy and that bacterial load is the key driver of sepsis [Lala HM et al. J Infect 2007]. Prof. Kumar suggested that the speed of clearance of the microbial pathogen is the critical determinant of outcome in septic shock.
Pneumococcal Pneumonia and Risk of Septic Shock.
Reprinted with permission from the American College of Chest Physicians. Rello J. Severity of pneumococal pneumonia associated with genomil bacteria load. Chest 2009; 136(3): 832.
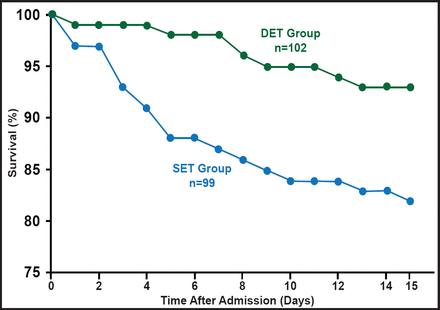
What then is the best approach for treatment? Prof. Kumar believes that early appropriate antimicrobial therapy is the simplest effective approach and has shown that early therapy is associated with significant improvement in mortality rates across a variety of clinical infections and microbes [Kumar A et al. Crit Care Med 2006]. “But what can you do if you miss the early window of opportunity?” asked Prof. Kumar. One option is to increase the intensity of the therapy by using a cidal versus static drug or increasing the dose to speed elimination of the pathogen. Another possible approach might be combination therapy (with drugs from different antiobiotic classes to which a pathogen is known to be sensitive), but monotherapy versus combination therapy studies show mixed results (Figure 2) [Safdar N et al. Lancet Infect Dis 2004; Micek S et al. Antimicrob Agents Chemother 2010].
Monotherapy Versus Combination Therapy in Severe Bacteremic Pneumococcal Pneumonia.
Reprinted with permission from A. Kumar, MD.
Among critically ill patients (but not less ill patients) with pneumococcal bacteremia, combination antibiotic therapy was associated with lower 14-day mortality (23.4% vs 55.3%; p=0.0015) [Baddour LM et al. Am J Respir Crit Care Med 2004]. Patients with community-acquired pneumonia with shock receiving combination therapy do better than those receiving monotherapy, but no difference is seen in patients not in shock [Rodríguez A et al. Crit Care Med 2007]. Prof. Kumar said that a meta-analysis study showed that combination antibiotic therapy yielded improved survival and clinical response of high-risk, life-threatening infections, particularly those associated with septic shock, but was detrimental to survival in low-risk patients [Kumar A et al. Crit Care Med 2010]. In another propensity-matched study of septic shock, the percentage of patients surviving at 28 days was greater for combination therapy compared with monotherapy (p=0.0002) across a broad range of clinical syndromes and pathogens [Kumar A et al. Crit Care Med 2010].
Prof. Kumar noted that there appears to be an underlying principle that explains divergent results in combination therapy, which implies that the benefits of combination therapy are primarily restricted to the critically ill, particularly those with shock, and that combination therapy is only required for short periods. In addition, combination therapy likely only makes sense when the combination of local antibiotic use patterns and local resistance/frequency distribution of pathogens results in suboptimal cidality with monotherapy.
- © 2012 MD Conference Express®