Summary
Extensively drug resistant Gram-negative superbugs Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae present a global medical challenge due to their resistance to almost all current antibiotics, and no new antibiotics will be available for many years to come. Frequently, the only active antibiotics currently in use are colistin (polymyxin E) and polymyxin B. This article presents an overview of how polymyxins kill Gram-negative superbugs and how resistance develops.
- Bacterial Infections
Extensively drug resistant (XDR) Gram-negative superbugs Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumoniae present a global medical challenge due to their resistance to almost all current antibiotics, and no new antibiotics will be available for many years to come. Frequently, the only active antibiotics currently in use are colistin (polymyxin E) and polymyxin B. Jian Li, PhD, Monash Institute of Pharmaceutical Sciences, Parkville, Victoria, Australia, presented an overview of how polymyxins kill Gram-negative superbugs and how resistance develops.
Polymyxins became available for clinical use more than 50 years ago but they were abandoned in the 1970s due to “high” rates of nephrotoxicity and neurotoxicity, and the availability of other new antibiotics. However, antibiotic resistance in Gram-negative superbugs has refocused attention on these agents over the last decade. Significant progress has been made recently in understanding the pharmacokinetics (PK) and pharmacodynamics (PD) of polymyxins. Polymyxins have a narrow spectrum and are active mostly against Gram-negative bacterial pathogens. They demonstrate rapid initial killing (but with the potential for regrowth) and negligible postantibiotic effect [Li J et al. Antimicrob Agents Chemother 2001, 2006; Dudhani RV et al. Antimicrob Agents Chemother 2010, J Antimicrob Chemother 2010]. Although the detailed mechanism of antibacterial activity of the polymyxins remains unclear, there is evidence that they rapidly permeabilize bacterial outer membrane by interacting with lipopolysaccharide (LPS) molecules [Hancock RE, Chappel DS. Antimicrob Agents Chemother 1999]. In vitro studies have shown that polymyxins can induce intermembrane molecular contacts. For example, in Escherichia coli metabolic changes leading to cell death are triggered when polymyxins induce phospholipid exchange between the outer membrane and cytoplasmic membrane, which forms envelope-crossing pores [Daugelavicius R et al. Antimicrob Agents Chemother 2000; Liechty A et al. Biochim Biophys Acta 2000]. A recent biochemical study suggests that polymyxins induce rapid killing of Gram-negative bacteria through hydroxyl radical production [Sampson TR et al. Antimicrob Agents Chemother 2012].
Pathogenic organisms have developed countermeasures to resist polymyxins. One study has shown that K. pneumoniae increased the production of capsule polysaccharide (CPS) when grown in the presence of polymyxin B. This suggests that CPS protects bacteria by limiting the interaction of antimicrobial peptides with bacterial surface [Campos MA et al. Infect Immun 2004]. Polymyxin resistance in A. baumannii can result from mutational inactivation of genes essential for lipid A biosynthesis [Moffatt JH et al. Antimicrob Agents Chemother 2010]. Strains harboring these mutations have been unable to produce LPS. In response to total LPS loss, A. baumannii alters the expression of critical transport and biosynthesis systems associated with modulating the composition and structure of the outer membrane [Henry R et al. Antimicrob Agents Chemother 2012]. In Gram-negative bacteria, the most common mechanism of polymyxin resistance is associated with modifications of lipid A of the LPS, thereby diminishing the initial polar interaction between positively charged polymyxins and negatively charged LPS [Moskowitz SM et al. Antimicrob Agents Chemother 2012; Arroyo LA et al. Antimicrob Agents Chemother 2011; Beceiro A et al. Antimicrob Agents Chemother 2011].
Dr. Li concluded by pointing out that understanding the mechanisms of activity and resistance of polymyxins will greatly help in the design of novel lipopeptides that are active against polymyxin-resistant MDR isolates.
Keith Kaye, MD, MPH, Wayne State University, Detroit, Michigan, USA, hypothesized that polymyxin combination therapy offers significant advantages for treating XDR Gram-negative bacilli; however, controlled data comparing combination therapy to monotherapy are sparse. He noted that combination therapy might prevent the emergence of resistance. Yet, clinical evidence for these advantages is very limited and collaborative investigator-initiated, multicenter trials are urgently needed [Paul M, Leibovici L. Infect Dis Clin North Am 2009].
Colistin is increasingly returning to use because its spectrum of activity focuses on Gram-negative organisms and it has no cross resistance with other classes of antibacterials. However, there are problems with colistin. It never underwent the rigorous development procedures that are required of modern antibiotics; therefore, there is a lack of conformity in dosing and the currently recommended dosing regimens are based on inaccurate PK data. Clinical experience with colistin varies greatly, making interpretation difficult. In addition, recent studies report dose-dependent nephrotoxicity in about 40% of all subjects [DeRyke CA et al. Antimicrob Agents Chemother 2010; Hartzell JD et al. Clin Infect Dis 2009; Pogue JM et al. Clin Infect Dis 2011].
Combination therapy with colistin is being considered for several reasons: colistin has shown synergistic effects with a variety of other agents in vitro, dose-related toxicity may limit colistin use in some situations, and, most importantly, the results of a study show treatment failure with colistin monotherapy in isolates that demonstrate heteroresistance to colistin [Rodriguez CH et al. Diagn Microbiol Infect Dis 2009]. Combination therapy might also prevent emergence of polymyxin resistance. Combinations of colistin plus rifampin, aminoglycosides, tigecycline, fosfomycin or carbapenems have been suggested and, so far, data have been encouraging. Several trials are underway with colistin combinations with rifampin, carbapenem, or fosfomycin. The role of lower dose colistin in combination therapy remains unclear.
Nebulization of colistin methanesulfonate (CMS), an inactive prodrug of colistin, is more efficient than intravenous administration of colistin to rapidly reach high efficient colistin concentrations within the lung, especially in intubated critical care patients with pulmonary infection, said William Couet, PhD, Université de Poitiers, Poitiers, France.
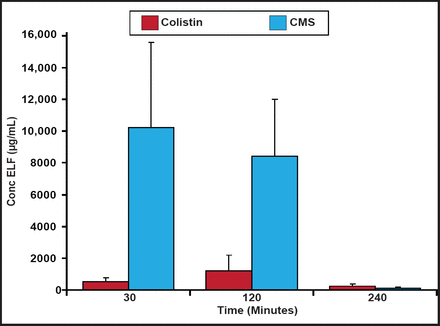
Plasma colistin concentrations at steady-state have been shown to be hardly higher than 2 µg/mL, which may be insufficient at treating some bacterial infections [Plachouras D et al. Antimicrob Agents Chemother 2009]; however, patients with ventilator-associated tracheobronchitis due to Gram-negative bacteria achieved relatively higher levels of colistin in the epithelial lining fluid (ELF) and favorable microbiological response using inhaled CMS [Athanassa ZE et al. Intensive Care Med 2012]. In a recent study with rats, two thirds of CMS was absorbed directly and one third was converted to colistin in the ELF following nebulization. CMS and formed colistin concentrations in the ELF after the inhalation of CMS (15 mg/kg in rats) are shown in Figure 1. Although lower in relative terms, colistin concentrations in the ELF could be high enough to show activity against microorganisms following CMS nebulization [Marchand S et al. Antimicrob Agents Chemother 2010].
CMS and Colistin Concentrations in ELF.
Reproduced with permission from the American Society for Microbiology. Marchand S et al. Aerosol therapy with colistin methanesulfonate: A biopharmaceutical issue illustrated in rats. Antimicrob Agents Chemother. 2010;54(9):3702–7.
Key characteristics of antibiotics for nebulization are low membrane permeability and, to a lower extent, efflux transport.
- © 2012 MD Conference Express®